True or False: For each of the following statement, state if it is true or false. If it is false, correct the statement. 10. A mole ratio can only be determined from a correctly balanced chemical equation.lond 21 Jan True (11, You must know the masses of all of the reactants in order to determine a theoretical mass of a product, even if a reactant is in excess. True 12. Coefficients are used when determining the molar mass of a compound. false, coefficients are used when determining the mole ratio 13. For any given chemical equation, all of the reactants will always be converted into products.xdi ei ist? (BET:MA)
True or False: For each of the following statement, state if it is true or false. If it is false, correct the statement. 10. A mole ratio can only be determined from a correctly balanced chemical equation.lond 21 Jan True (11, You must know the masses of all of the reactants in order to determine a theoretical mass of a product, even if a reactant is in excess. True 12. Coefficients are used when determining the molar mass of a compound. false, coefficients are used when determining the mole ratio 13. For any given chemical equation, all of the reactants will always be converted into products.xdi ei ist? (BET:MA)
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 41QAP: Which of the following statements is(are) true? l type='a'> A balanced equation relates the numbers...
Related questions
Question
100%
Is number 11 a true or false statement and why? this is a non graded practice worksheet
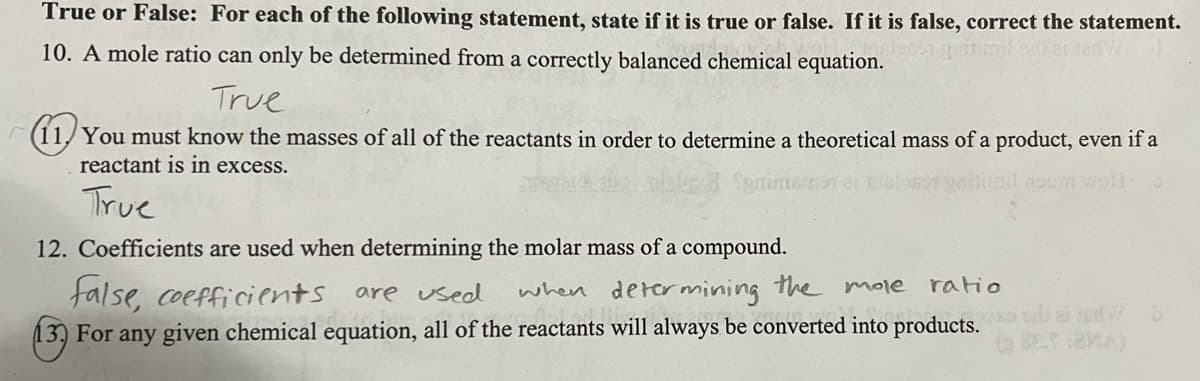
Transcribed Image Text:True or False: For each of the following statement, state if it is true or false. If it is false, correct the statement.
10. A mole ratio can only be determined from a correctly balanced chemical equation.
True
(11,You must know the masses of all of the reactants in order to determine a theoretical mass of a product, even if a
reactant is in excess.
trail doom wo:
True
12. Coefficients are used when determining the molar mass of a compound.
false, coefficients are used
when determining the mole ratio
13) For any given chemical equation, all of the reactants will always be converted into products. aid b
(BET:MA)
Expert Solution
Step 1
Answer:
Balancing of a chemical equation is based on the law of conservation of mass which suggests that atom of each type should be in equal number on both sides of the chemical equation because then only total mass of reactants will be equal to the total mass of products.
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning