The equilibrium for the various proportions of the amyl ester of dichloroacetic acid (CHCI, COOC,H) into the acid (CHOCI,COOH) and amylene (C,Hiol was investigated. The initial amount for the CHCI,COOC,H used is 1 mole. Complete the table below: Let b the iniial moles of amylene. V the total volume of mixtures in liters, x be the number of moles of V. Kc 1.05 0.215 0.455 0.401 3.12 ester at equilibrium and Kc 2.61 equilibrium constant in moles per liter. 4.45 0.628 3.54 0.794 0.658 3.44
The equilibrium for the various proportions of the amyl ester of dichloroacetic acid (CHCI, COOC,H) into the acid (CHOCI,COOH) and amylene (C,Hiol was investigated. The initial amount for the CHCI,COOC,H used is 1 mole. Complete the table below: Let b the iniial moles of amylene. V the total volume of mixtures in liters, x be the number of moles of V. Kc 1.05 0.215 0.455 0.401 3.12 ester at equilibrium and Kc 2.61 equilibrium constant in moles per liter. 4.45 0.628 3.54 0.794 0.658 3.44
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter10: Solutions
Section: Chapter Questions
Problem 26QAP
Related questions
Question
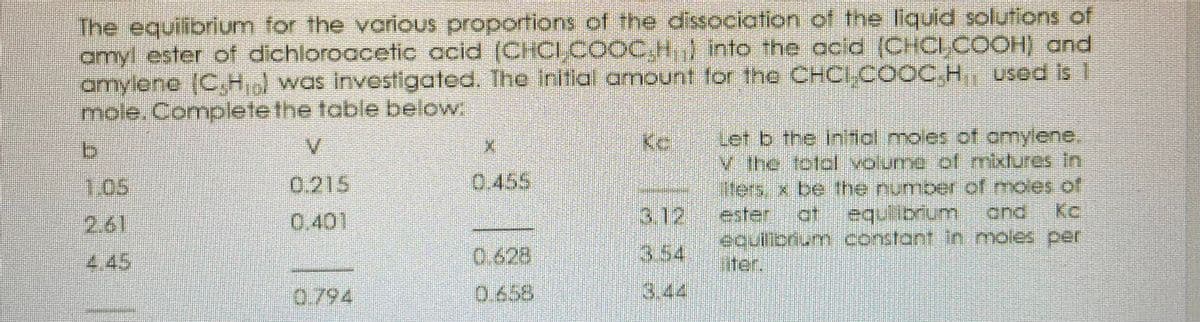
Transcribed Image Text:The equilibrium for the vorious proportions of the dissociation of the liquid solutions of
amyl ester of dichloroacetic ccid (CHCI,.COOC,H,} into the acid (CHCLCOOH) and
amylene (C,H was investigated. The inltial amount tor the CHCI,COOC.H used Is 1
mole. Completethe table below
Let b the inltial moles of omylene,
V the totdl volume cof mixtures in
iters, x. be the number of moles of
V.
1.05
0.215
0.455
3.12
.af
equibrium
ond
Kc
ester
outionum constont n.moles per
iter.
2.61
0.401
4.45
0.628
3.54
0.794
0.658
3.44
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning