The experimental values of the dipole moments of the chlorobenzene and nitrobenzene molecules are: 5.27x10 30 and 1.33x10 29 C m, respectively. From these data, obtain an estimate of the dipole moments of the following molecules: a) 1-chloro-3-nitrobenzene (experimental value = 1.244x1029 C m) b) 1-chloro-4-nitrobenzene (experimental value = 9.440x10-30 C m) c) 1,3-dichloro-5-nitrobenzene (experimental value = 8.940x10 30C m) Discuss the results obtained.
The experimental values of the dipole moments of the chlorobenzene and nitrobenzene molecules are: 5.27x10 30 and 1.33x10 29 C m, respectively. From these data, obtain an estimate of the dipole moments of the following molecules: a) 1-chloro-3-nitrobenzene (experimental value = 1.244x1029 C m) b) 1-chloro-4-nitrobenzene (experimental value = 9.440x10-30 C m) c) 1,3-dichloro-5-nitrobenzene (experimental value = 8.940x10 30C m) Discuss the results obtained.
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter20: Dienes, Conjugated Systems, And Pericyclic Reactions
Section20.3: Uv-visible Spectroscopy
Problem 20.5P
Related questions
Question
100%
Image
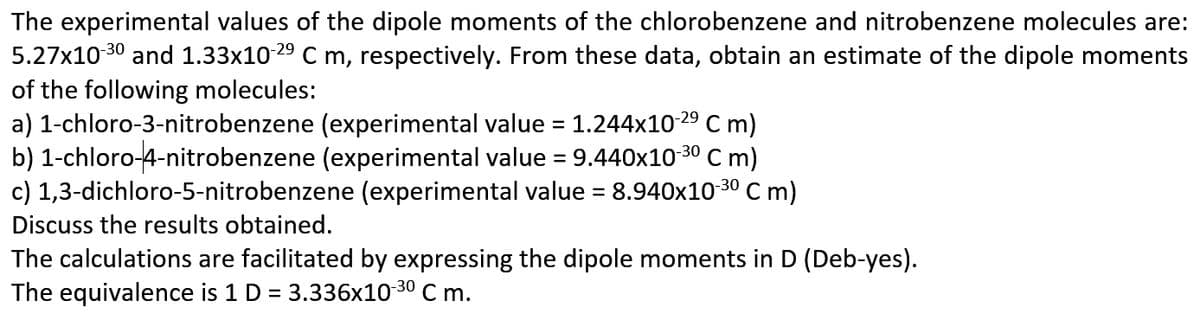
Transcribed Image Text:The experimental values of the dipole moments of the chlorobenzene and nitrobenzene molecules are:
5.27x10 30 and 1.33x10 29 C m, respectively. From these data, obtain an estimate of the dipole moments
of the following molecules:
a) 1-chloro-3-nitrobenzene (experimental value = 1.244x10 29 C m)
b) 1-chloro-4-nitrobenzene (experimental value = 9.440x10
c) 1,3-dichloro-5-nitrobenzene (experimental value = 8.940x10 30 Cm)
C m)
30
Discuss the results obtained.
The calculations are facilitated by expressing the dipole moments in D (Deb-yes).
The equivalence is 1 D = 3.336x10-30 C m.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning