The figure below illustrates a few thermodynamic processes, distinguished by the vertices of the paths. p (atm) 4 4.0 3.0 - C 2.0 - 1.0 - A 1.0 5.0 V (L) 2.0 3.0 4.0 Answer the questions below. a. Calculate the work done by the gas in quasi-static processes renresented by each of the nathr (ll
The figure below illustrates a few thermodynamic processes, distinguished by the vertices of the paths. p (atm) 4 4.0 3.0 - C 2.0 - 1.0 - A 1.0 5.0 V (L) 2.0 3.0 4.0 Answer the questions below. a. Calculate the work done by the gas in quasi-static processes renresented by each of the nathr (ll
Physics for Scientists and Engineers with Modern Physics
10th Edition
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter20: The Kinetic Theory Of Gases
Section: Chapter Questions
Problem 34AP: In a cylinder, a sample of an ideal gas with number of moles n undergoes an adiabatic process. (a)...
Related questions
Question
100%
Please answer part A
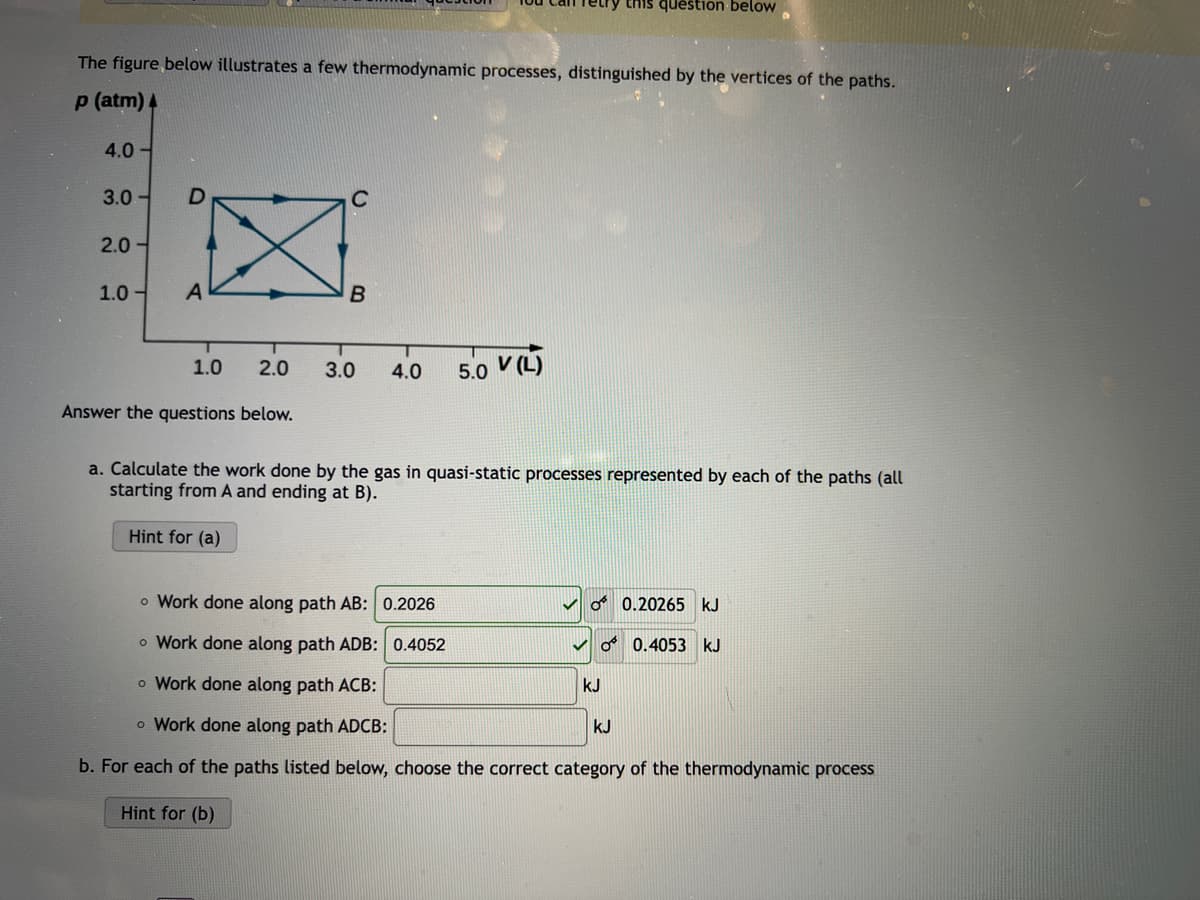
Transcribed Image Text:etry this question below
The figure below illustrates a few thermodynamic processes, distinguished by the vertices of the paths.
p (atm) 4
4.0 -
3.0 -
2.0 -
1.0 -
5.0 V(L)
1.0
2.0
3.0
4.0
Answer the questions below.
a. Calculate the work done by the gas in quasi-static processes represented by each of the paths (all
starting from A and ending at B).
Hint for (a)
o Work done along path AB: 0.2026
O 0.20265 kJ
o Work done along path ADB: 0.4052
O 0.4053 kJ
o Work done along path ACB:
kJ
o Work done along path ADCB:
kJ
b. For each of the paths listed below, choose the correct category of the thermodynamic process
Hint for (b)
Expert Solution
Step 1
Work done in thermodynamic process is the area bounded between curve and volume axis.
1 L = 10-3 m3
1 atm = 1.013 × 105 Pa
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning