The figure below is the basic plot of pressure vs. volume for the Dual Standard Cycle. Constant pressure heat addition 3 4 PV = Pressure (P) Heat IN = constant Adiabatic expansion 2 5 Heat OUT (Constant volume heat rejection) Constant volume heat addition 1 Adiabatic compression Volume (V) Using the Dual Air Standard Cycle and the following data: • Assume constant properties of air. R = 287 J/kgK; Cp = 1005 J/kgK; Cv = 718 J/kgK; y = 1.4 • Cycle compression ratio = 14 (V₁/V₂) • Air conditions prior to compression: Pressure = 1.3 bar; Temperature = 293K • Heat added at constant volume = 280000 J/kg • Heat added at constant pressure = 662000 J/kg
The figure below is the basic plot of pressure vs. volume for the Dual Standard Cycle. Constant pressure heat addition 3 4 PV = Pressure (P) Heat IN = constant Adiabatic expansion 2 5 Heat OUT (Constant volume heat rejection) Constant volume heat addition 1 Adiabatic compression Volume (V) Using the Dual Air Standard Cycle and the following data: • Assume constant properties of air. R = 287 J/kgK; Cp = 1005 J/kgK; Cv = 718 J/kgK; y = 1.4 • Cycle compression ratio = 14 (V₁/V₂) • Air conditions prior to compression: Pressure = 1.3 bar; Temperature = 293K • Heat added at constant volume = 280000 J/kg • Heat added at constant pressure = 662000 J/kg
Elements Of Electromagnetics
7th Edition
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Sadiku, Matthew N. O.
ChapterMA: Math Assessment
Section: Chapter Questions
Problem 1.1MA
Related questions
Question
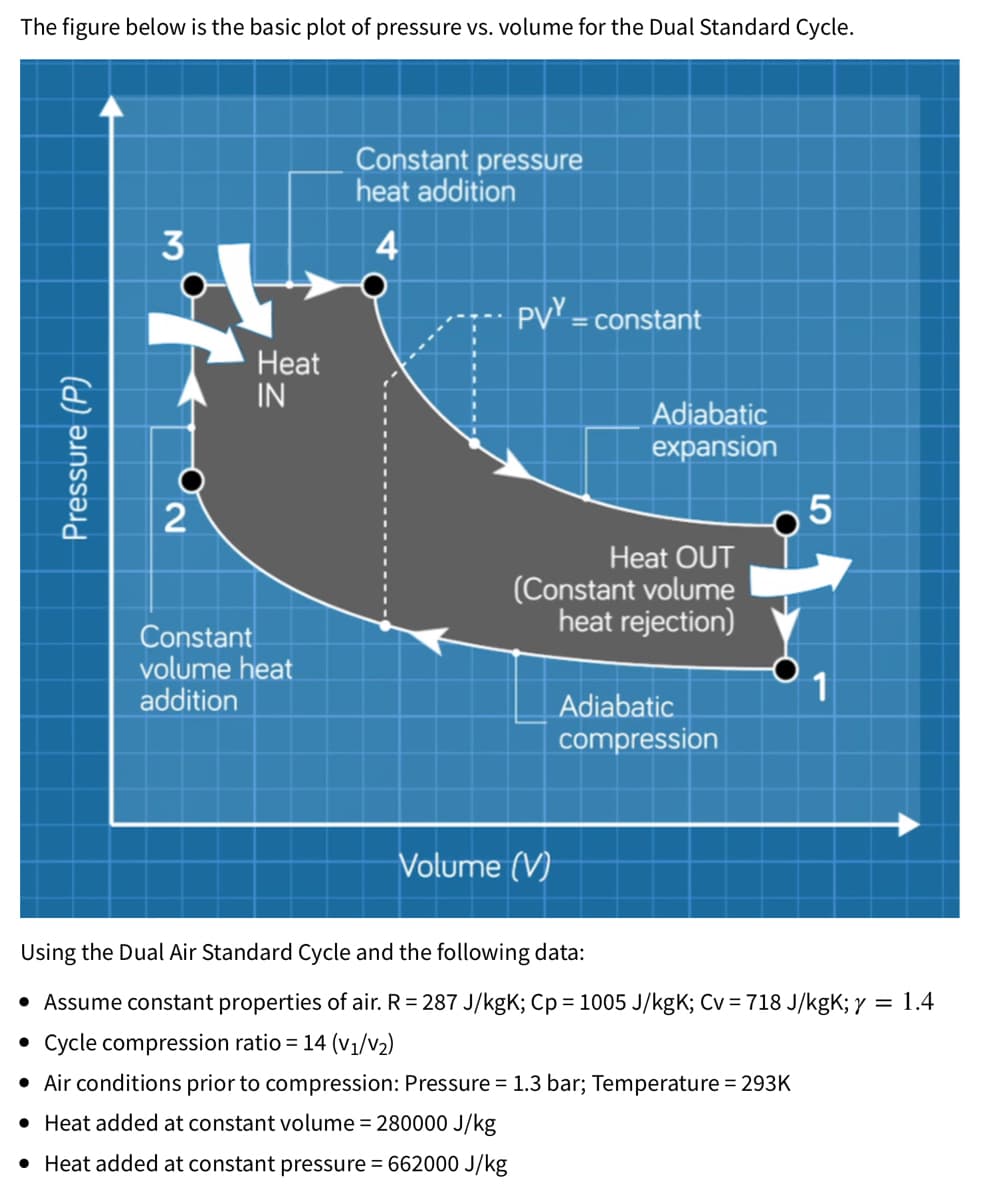
Transcribed Image Text:The figure below is the basic plot of pressure vs. volume for the Dual Standard Cycle.
Constant pressure
heat addition
3
4
PV = constant
%3D
Heat
IN
Adiabatic
expansion
2
Heat OUT
(Constant volume
heat rejection)
Constant
volume heat
addition
1
Adiabatic
compression
Volume (V)
Using the Dual Air Standard Cycle and the following data:
• Assume constant properties of air. R= 287 J/kgK; Cp = 1005 J/kgK; Cv = 718 J/kgK; y = 1.4
• Cycle compression ratio = 14 (V1/v2)
• Air conditions prior to compression: Pressure = 1.3 bar; Temperature = 293K
• Heat added at constant volume = 280000 J/kg
• Heat added at constant pressure = 662000 J/kg
Pressure (P)

Transcribed Image Text:a)
Calculate the pressure at the end of the compression process.
P2 =
bar
b)
Calculate the temperature at the end of the compression process.
T2 =
K
c)
Calculate the temperature at the end of the constant volume heat addition process.
T3 =
K
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:
9781118170519
Author:
Norman S. Nise
Publisher:
WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:
9781337093347
Author:
Barry J. Goodno, James M. Gere
Publisher:
Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:
9781118807330
Author:
James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:
WILEY