The figure below shows a portion of a GC chromatogram for a mixture of two aromatic compounds labeled A and B. The separation employed a 2.50 meter packed column under isothermal conditions (90 °C) and a flow rate of 10 mL/min.
The figure below shows a portion of a GC chromatogram for a mixture of two aromatic compounds labeled A and B. The separation employed a 2.50 meter packed column under isothermal conditions (90 °C) and a flow rate of 10 mL/min.
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter26: An Introduction To Chromatographic Separations
Section: Chapter Questions
Problem 26.14QAP
Related questions
Question
questioon f only
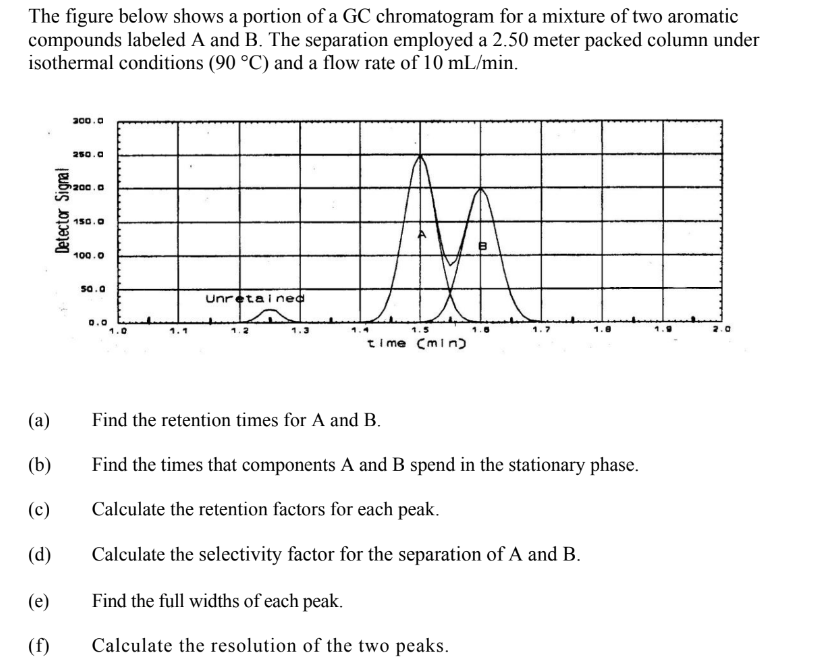
Transcribed Image Text:The figure below shows a portion of a GC chromatogram for a mixture of two aromatic
compounds labeled A and B. The separation employed a 2.50 meter packed column under
isothermal conditions (90 °C) and a flow rate of 10 mL/min.
(a)
(b)
(c)
(d)
(e)
(f)
Detector Signal
300.0
250.0
200.0
150.0
100.0
50.0
0.0
1.0
Unretained
1.2
1.5
time (min)
Find the retention times for A and B.
Find the times that components A and B spend in the stationary phase.
Calculate the retention factors for each peak.
Calculate the selectivity factor for the separation of A and B.
Find the full widths of each peak.
Calculate the resolution of the two peaks.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

