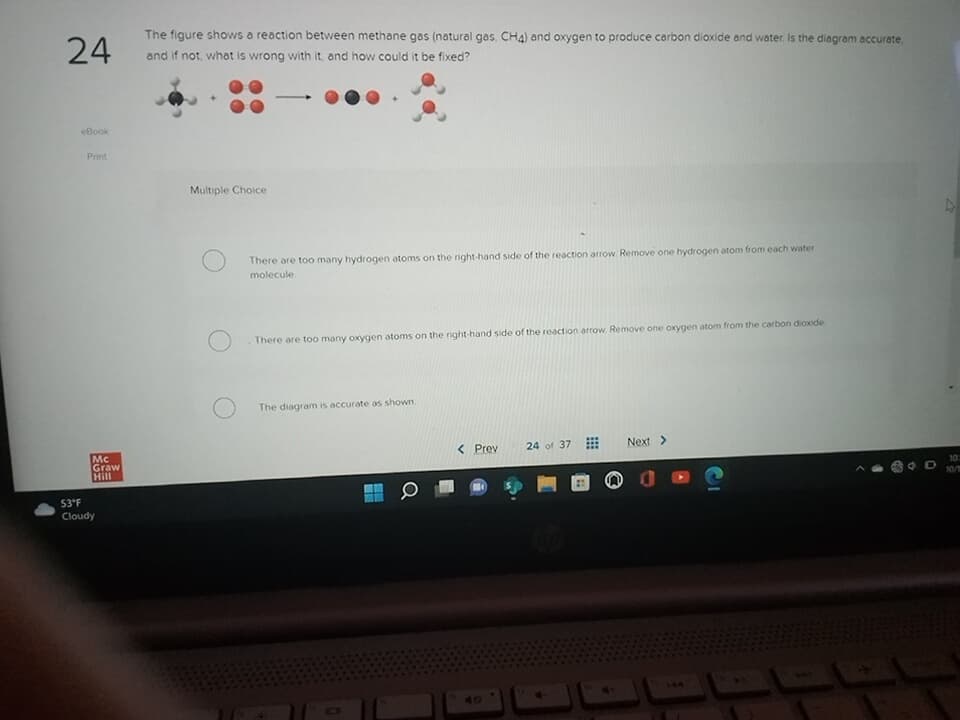
The figure shows a reaction between methane gas (natural gas, CH4) and oxygen to produce carbon dioxide and water. Is the diagram accurate and if not, what is wrong with it, and how could it be fixed? Multiple Choice - There are too many hydrogen atoms on the right-hand side of the reaction arrow Remove one hydrogen atom from each water molecule There are too many oxygen atoms on the right-hand side of the reaction arrow Remove one oxygen atom from the carbon dioxide The diagram is accurate as shown
The figure shows a reaction between methane gas (natural gas, CH4) and oxygen to produce carbon dioxide and water. Is the diagram accurate and if not, what is wrong with it, and how could it be fixed? Multiple Choice - There are too many hydrogen atoms on the right-hand side of the reaction arrow Remove one hydrogen atom from each water molecule There are too many oxygen atoms on the right-hand side of the reaction arrow Remove one oxygen atom from the carbon dioxide The diagram is accurate as shown
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter19: The Representative Elements
Section: Chapter Questions
Problem 104CP
Related questions
Question
The figure shows a reaction between methane gas and oxygen to produce carbon dioxide and water . Is the diagram accurate.
Transcribed Image Text:24
eBook
Print
Mc
Graw
Hill
53°F
Cloudy
The figure shows a reaction between methane gas (natural gas. CH4) and oxygen to produce carbon dioxide and water. Is the diagram accurate,
and if not, what is wrong with it, and how could it be fixed?
Multiple Choice
-
There are too many hydrogen atoms on the right-hand side of the reaction arrow Remove one hydrogen atom from each water
molecule
There are too many oxygen atoms on the right-hand side of the reaction arrow Remove one oxygen atom from the carbon dioxide
The diagram is accurate as shown.
< Prev
24 of 37
Next >
OD
10
10/1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning