The Find the minimum energy needed to eject electrons from a metal with a threshold frequency of 3.09 x 1014 s-. E, = With what maximum kinetic energy will electrons be ejected when this metal is exposed to light with a wavelength of 285 nm? KEelectron =
The Find the minimum energy needed to eject electrons from a metal with a threshold frequency of 3.09 x 1014 s-. E, = With what maximum kinetic energy will electrons be ejected when this metal is exposed to light with a wavelength of 285 nm? KEelectron =
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter4: Introduction To Quantum Mechanics
Section: Chapter Questions
Problem 50AP
Related questions
Question
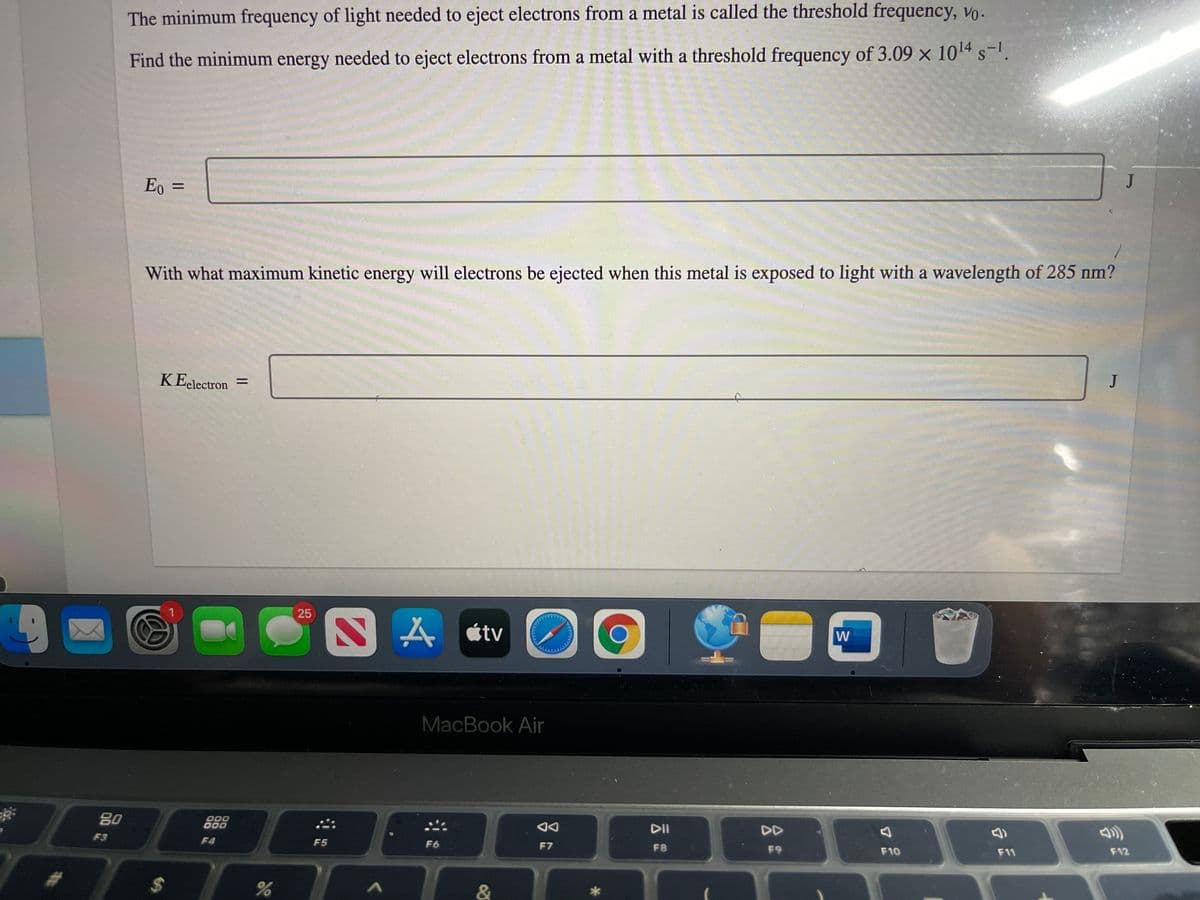
Transcribed Image Text:The minimum frequency of light needed to eject electrons from a metal is called the threshold frequency, vo.
Find the minimum energy needed to eject electrons from a metal with a threshold frequency of 3.09 x 1014 s-.
Eo =
J
With what maximum kinetic energy will electrons be ejected when this metal is exposed to light with a wavelength of 285 nm?
KEelectron
J
%3D
1
A étv
W
MacBook Air
80
DO0
D00
DI
DD
F3
F4
F5
F6
F7
F8
F9
F10
F11
F12
%24
&
25
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning