The following equation describes the equilibrium that exists when the weak acid propionic acid (C2 H; CO,H) dissolves in water. (In acting as an acid, the -CO2H group supplies Ht to form H30*.) C2 H; CO2H(aq) + H2O(8) 2 H30+ (aq) + C2H5 CO2 (aq) Which of the four species are Bronsted-Lowry acids? (Select all that apply.) O H20 O C,H; CO2- O H;0* O C2H5 CO,H b Which of the four species are Brønsted-Lowry bases? (Select all that apply.) O H2O O C;H; CO2- O H;0+ O C;H; CO,H
The following equation describes the equilibrium that exists when the weak acid propionic acid (C2 H; CO,H) dissolves in water. (In acting as an acid, the -CO2H group supplies Ht to form H30*.) C2 H; CO2H(aq) + H2O(8) 2 H30+ (aq) + C2H5 CO2 (aq) Which of the four species are Bronsted-Lowry acids? (Select all that apply.) O H20 O C,H; CO2- O H;0* O C2H5 CO,H b Which of the four species are Brønsted-Lowry bases? (Select all that apply.) O H2O O C;H; CO2- O H;0+ O C;H; CO,H
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter27: Amines
Section: Chapter Questions
Problem 10E
Related questions
Question
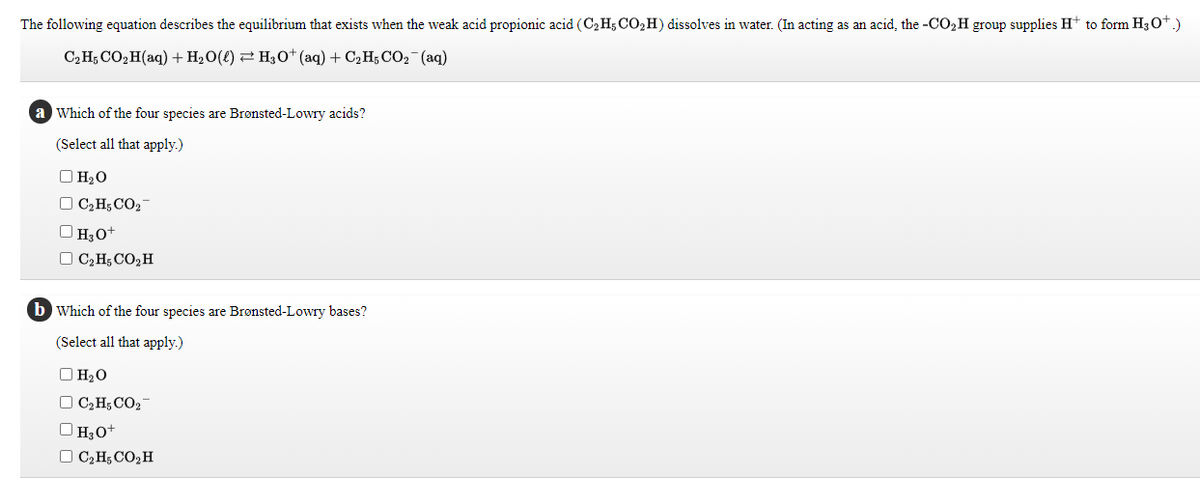
Transcribed Image Text:The following equation describes the equilibrium that exists when the weak acid propionic acid (C2H5 CO,H) dissolves in water. (In acting as an acid, the -CO2H group supplies H+ to form H30*.)
C2H; CO,H(aq) +H2O(l) 2 H,0+ (aq) + C2 H; CO2-(aq)
a Which of the four species are Bronsted-Lowry acids?
(Select all that apply.)
O H20
O C2H; CO2-
O H3o+
O C2H; CO,H
b Which of the four species are Brønsted-Lowry bases?
(Select all that apply.)
O H2O
O C2H;CO2
O H30+
O C;H; CO,H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning