The following facts are known about the two reactions: • Reaction 1 is exothermic and Reaction 2 is endothermic. • If a reaction vessel is charged ("filled") with A and B, then at first C is produced faster than D. Use these facts to sketch a qualitative reaction energy diagram for both reactions. Note: because these sketches are only qualitative, the energies don't have to be exact. They only have to have the right relationship to each other. if one energy is less than another, that fact should be clear in your sketch.
The following facts are known about the two reactions: • Reaction 1 is exothermic and Reaction 2 is endothermic. • If a reaction vessel is charged ("filled") with A and B, then at first C is produced faster than D. Use these facts to sketch a qualitative reaction energy diagram for both reactions. Note: because these sketches are only qualitative, the energies don't have to be exact. They only have to have the right relationship to each other. if one energy is less than another, that fact should be clear in your sketch.
General, Organic, and Biological Chemistry
7th Edition
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:H. Stephen Stoker
Chapter9: Chemical Reactions
Section: Chapter Questions
Problem 9.21EP: Classify each of the following reactions as (1) a redox reaction (2) a nonredox reaction or (3) cant...
Related questions
Question
So,i do not understand nothing. On how to this. Please explain with steps,and also, can you mark how many times it went up and down,the limit is 8 steps going up.
Thanks
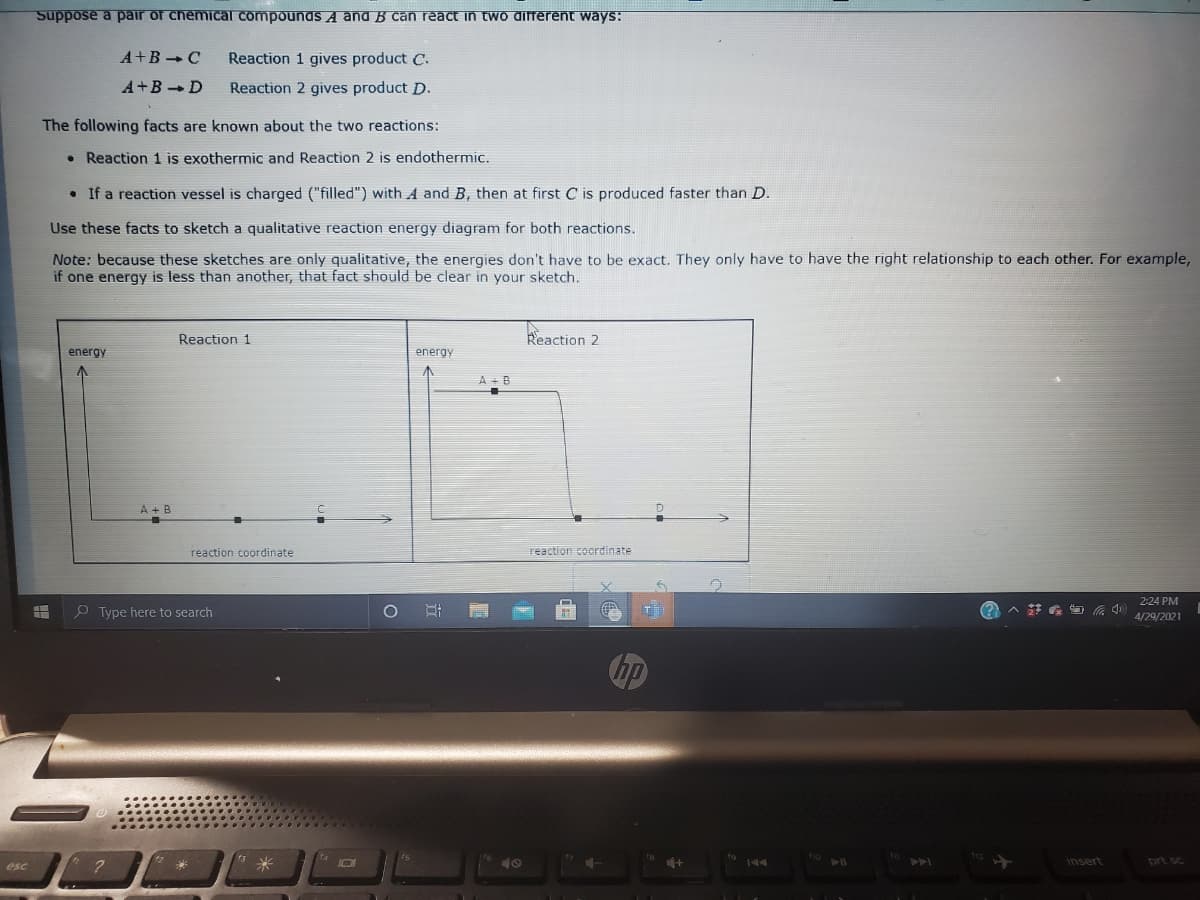
Transcribed Image Text:Suppose a pair of chemical compounas A and B can react in two different ways:
A+B C
Reaction 1 gives product C.
A+BD
Reaction 2 gives product D.
The following facts are known about the two reactions:
• Reaction 1 is exothermic and Reaction 2 is endothermic.
• If a reaction vessel is charged ("filled") with A and B, then at first C is produced faster than D.
Use these facts to sketch a qualitative reaction energy diagram for both reactions.
Note: because these sketches are only qualitative, the energies don't have to be exact. They only have to have the right relationship to each other. For example,
if one energy is less than another, that fact should be clear in your sketch.
Reaction 1
Reaction 2
energy
energy
A + B
A + B
reaction coordinate
reaction coordinate
2:24 PM
O Type here to search
(?
ヘ 口
4/29/2021
hp
米
insert
prt sc
144
esc
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning