Base your answers to practice questions 43 and 44 on the information below. Given the reaction: 2NO2(g) + 7H2(g) 2NH3(g) + 4H20(g) + 1127 kJ 43. On the diagram to the left, complete the potential energy diagram for this reaction. 2NO (g) + 7H,(g) 44. Determine the total amount of energy released when 0.5 moles of NO2 is completely reacted with hydrogen. Reaction Coordinate 45. On the potential energy diagram below, draw an arrow to represent the activation energy of the forward reaction. Reaction Coordinate 46. A potential energy diagram for a chemical reaction is shown below. On this diagram, use a dash-line to draw a curve to show how the potential energy diagram will change when a catalyst is added to the reaction. Reaction Coordinate Potential Energy Potential Energy Potential Energy
Base your answers to practice questions 43 and 44 on the information below. Given the reaction: 2NO2(g) + 7H2(g) 2NH3(g) + 4H20(g) + 1127 kJ 43. On the diagram to the left, complete the potential energy diagram for this reaction. 2NO (g) + 7H,(g) 44. Determine the total amount of energy released when 0.5 moles of NO2 is completely reacted with hydrogen. Reaction Coordinate 45. On the potential energy diagram below, draw an arrow to represent the activation energy of the forward reaction. Reaction Coordinate 46. A potential energy diagram for a chemical reaction is shown below. On this diagram, use a dash-line to draw a curve to show how the potential energy diagram will change when a catalyst is added to the reaction. Reaction Coordinate Potential Energy Potential Energy Potential Energy
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter4: Energy And Chemical Reactions
Section4.2: Conservation Of Energy
Problem 4.2CE
Related questions
Question
Hello, is it possible to answer all of them since they're multiple choice? Would really appreciate it. Thank you.
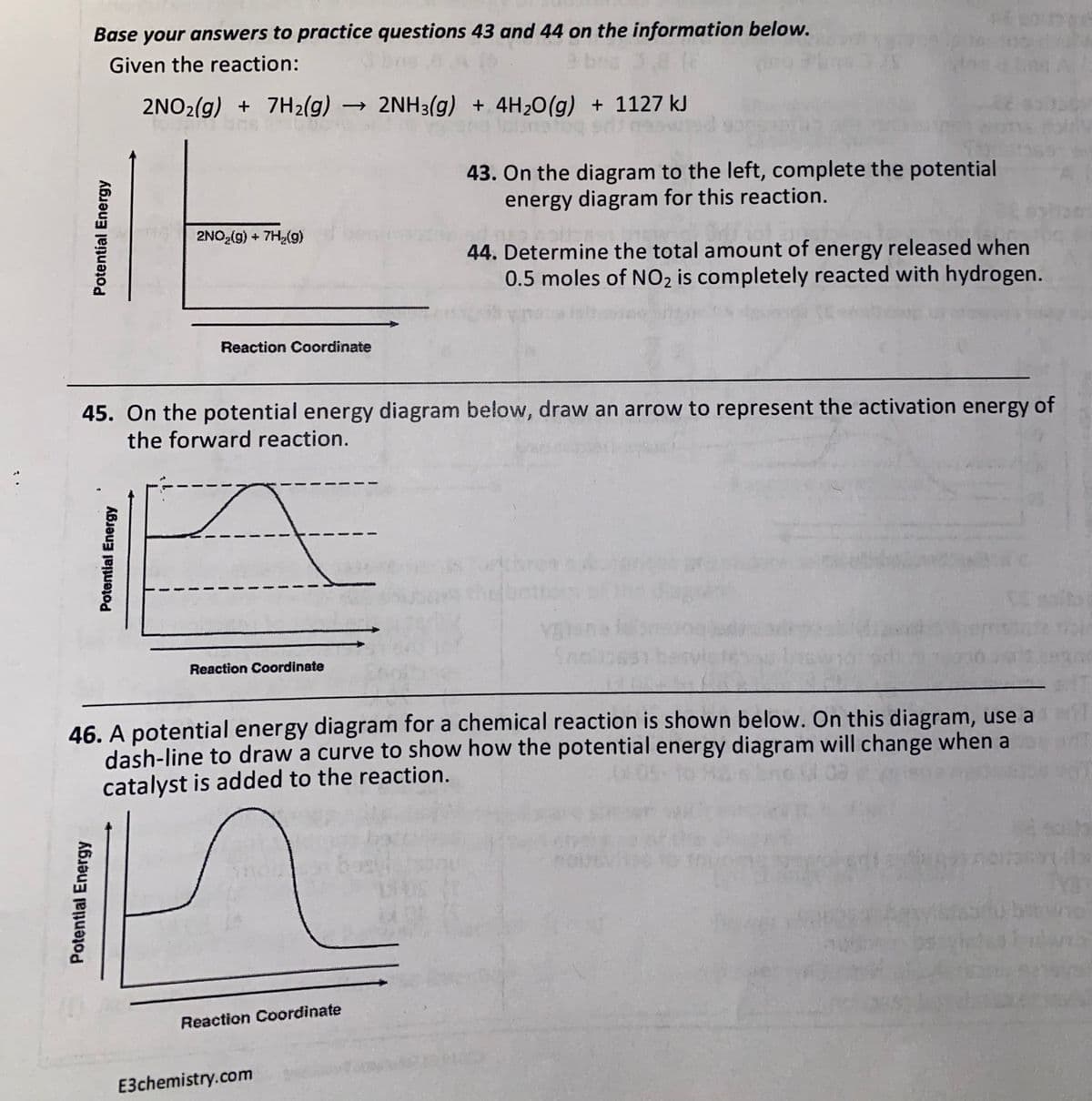
Transcribed Image Text:Base your answers to practice questions 43 and 44 on the information below.
Given the reaction:
2NO2(g) + 7H2(g) → 2NH3(g) + 4H20(g) + 1127 kJ
43. On the diagram to the left, complete the potential
energy diagram for this reaction.
2NO2(g) + 7H,(9)
44. Determine the total amount of energy released when
0.5 moles of NO2 is completely reacted with hydrogen.
Reaction Coordinate
45. On the potential energy diagram below, draw an arrow to represent the activation energy of
the forward reaction.
cusRA
YST
Reaction Coordinate
46. A potential energy diagram for a chemical reaction is shown below. On this diagram, use a
dash-line to draw a curve to show how the potential energy diagram will change when a
catalyst is added to the reaction.
Reaction Coordinate
E3chemistry.com
Potential Energy
Potential Energy
Potential Energy
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER
