The following information is given for cadmium at latm: AHvap (765.00°C) = 889.6 J/g AHtu(321.00°C) = 54.40 J/g T = 765.00°C Tm = 321.00°C Specific heat solid = 0.2300 J/g °C Specific heat liquid = 0.2640 J/g °C A 49.50 g sample of liquid cadmium at 382.00°C is poured into a mold and allowed to cool to 23.00°C. How many kJ of energy are released in this process? (Report the answer as a positive number.) Energy = kJ
The following information is given for cadmium at latm: AHvap (765.00°C) = 889.6 J/g AHtu(321.00°C) = 54.40 J/g T = 765.00°C Tm = 321.00°C Specific heat solid = 0.2300 J/g °C Specific heat liquid = 0.2640 J/g °C A 49.50 g sample of liquid cadmium at 382.00°C is poured into a mold and allowed to cool to 23.00°C. How many kJ of energy are released in this process? (Report the answer as a positive number.) Energy = kJ
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter4: Energy And Chemical Reactions
Section4.5: Energy And Enthalpy
Problem 4.6PSP
Related questions
Question
please explain
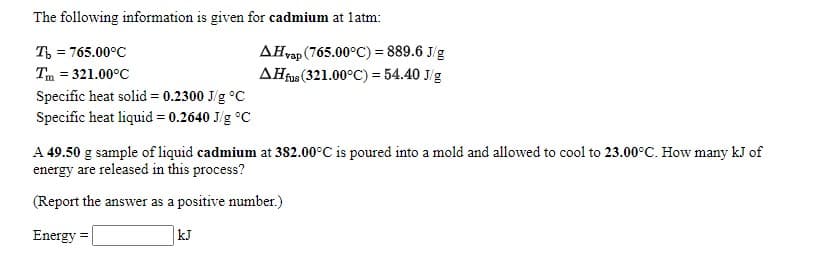
Transcribed Image Text:The following information is given for cadmium at latm:
AHvap (765.00°C) = 889.6 J/g
AHfus (321.00°C) = 54.40 J/g
T, = 765.00°C
Tm = 321.00°C
Specific heat solid = 0.2300 J/g °C
Specific heat liquid = 0.2640 J/g °C
A 49.50 g sample of liquid cadmium at 382.00°C is poured into a mold and allowed to cool to 23.00°C. How many kJ of
energy are released in this process?
(Report the answer as a positive number.)
Energy
kJ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning