Suppose you want to build a fancy supercomputer. The machinery will produce a lot of heat, so you need to build a special cooling system. Given the specific heat of the following substances, explain which would be the best for cooling your supercomputer and why. Table of Selected Specific Heats 2.36 (kJ kg² K²) 4.19 (kJ kg² K²) 0.14 (kJ kg² K'). 1.97 (kJ kg² K³). Ethylene glycol Water Mercury Soy bean oil
Suppose you want to build a fancy supercomputer. The machinery will produce a lot of heat, so you need to build a special cooling system. Given the specific heat of the following substances, explain which would be the best for cooling your supercomputer and why. Table of Selected Specific Heats 2.36 (kJ kg² K²) 4.19 (kJ kg² K²) 0.14 (kJ kg² K'). 1.97 (kJ kg² K³). Ethylene glycol Water Mercury Soy bean oil
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 26E: When 50.0 g of 0.200 M NaCl(aq) at 24.1 C is added to 100.0 g of 0.100 M AgNO3(aq) at 24.1 C in a...
Related questions
Question
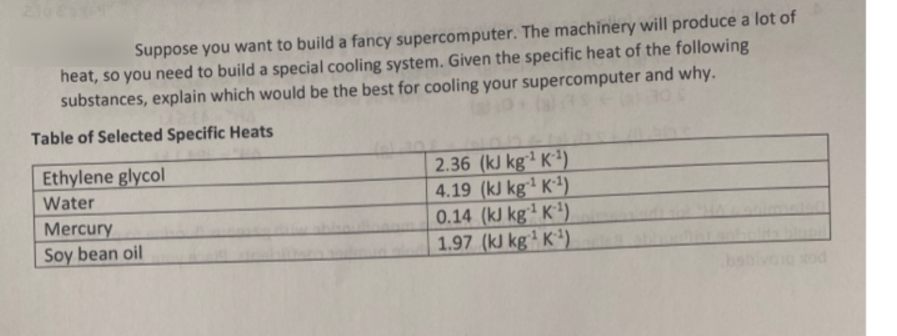
Transcribed Image Text:Suppose you want to build a fancy supercomputer. The machinery will produce a lot of
heat, so you need to build a special cooling system. Given the specific heat of the following
substances, explain which would be the best for cooling your supercomputer and why.
Table of Selected Specific Heats
2.36 (kJ kg² K²)
4.19 (kJ kg² K²)
0.14 (kJ kg² K').
1.97 (kJ kg² K³).
Ethylene glycol
Water
Mercury
Soy bean oil
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning