The following table shows the structures of some organic molecules together with their pKa values. Write the ionization reaction, they must take into account the hydrogen that is given off, the hydrogen acid. What is the strongest acid? Propose an explanation to justify the difference in acidity values
The following table shows the structures of some organic molecules together with their pKa values. Write the ionization reaction, they must take into account the hydrogen that is given off, the hydrogen acid. What is the strongest acid? Propose an explanation to justify the difference in acidity values
Chapter3: Mechanisms
Section: Chapter Questions
Problem 133EQ
Related questions
Question
100%
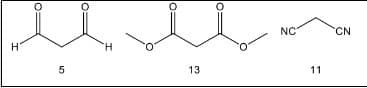
The following table shows the structures of some organic molecules together with their pKa values.
Write the ionization reaction, they must take into account the hydrogen that is given off, the hydrogen acid.
What is the strongest acid? Propose an explanation to justify the difference in acidity values.
Transcribed Image Text:NC
CN
H.
13
11
エ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning