The gas-phase reaction between methanol (A) and acetic acid (B) to form methyl acetate (C) and water (D) takes place in a batch reactor and proceeds to equilibrium. When the reactor mixture comes to equilibrium, the mole fractions of the four reactive species are related by a reaction equilibrium constant The feed to the reactor consists of nÃO, nвo, nco, ndo and nio gram moles of A, B, C, D and an inert gas I respectively. What is the composition of the final product? Yacetic acid: a. If nÃO = 0.600 ngo, and all other input quantities are 0.0, what is the equilibrium fractional conversion of A? i .46 Ymethylacetate: b. It is desired to produce 70.0 moles of methyl acetate starting with 73.0 moles of methanol, an unknown amount of acetic acid, and no other components. Ymethanol: If the reaction proceeds to equilibrium, how much acetic acid must be fed? Required acetic acid: 639 mol Ywater: CH3OH + CH3COOH=CH₂OOCCH3 + H₂O 1888 Extent: Required methanol: Required acetic acid: i YC YD YA YB c. Suppose it were important to reduce the concentration of methanol by making its conversion at equilibrium 98.0%. Again, assuming the feed to the reactor contains only methanol and acetic acid and that it is desired to produce 70.0 moles of methyl acetate, determine the extent of reaction and moles of methanol and acetic acid that must be fed to the reactor. .462 = i 639 Keg = 2.87 mol mol
The gas-phase reaction between methanol (A) and acetic acid (B) to form methyl acetate (C) and water (D) takes place in a batch reactor and proceeds to equilibrium. When the reactor mixture comes to equilibrium, the mole fractions of the four reactive species are related by a reaction equilibrium constant The feed to the reactor consists of nÃO, nвo, nco, ndo and nio gram moles of A, B, C, D and an inert gas I respectively. What is the composition of the final product? Yacetic acid: a. If nÃO = 0.600 ngo, and all other input quantities are 0.0, what is the equilibrium fractional conversion of A? i .46 Ymethylacetate: b. It is desired to produce 70.0 moles of methyl acetate starting with 73.0 moles of methanol, an unknown amount of acetic acid, and no other components. Ymethanol: If the reaction proceeds to equilibrium, how much acetic acid must be fed? Required acetic acid: 639 mol Ywater: CH3OH + CH3COOH=CH₂OOCCH3 + H₂O 1888 Extent: Required methanol: Required acetic acid: i YC YD YA YB c. Suppose it were important to reduce the concentration of methanol by making its conversion at equilibrium 98.0%. Again, assuming the feed to the reactor contains only methanol and acetic acid and that it is desired to produce 70.0 moles of methyl acetate, determine the extent of reaction and moles of methanol and acetic acid that must be fed to the reactor. .462 = i 639 Keg = 2.87 mol mol
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
100%
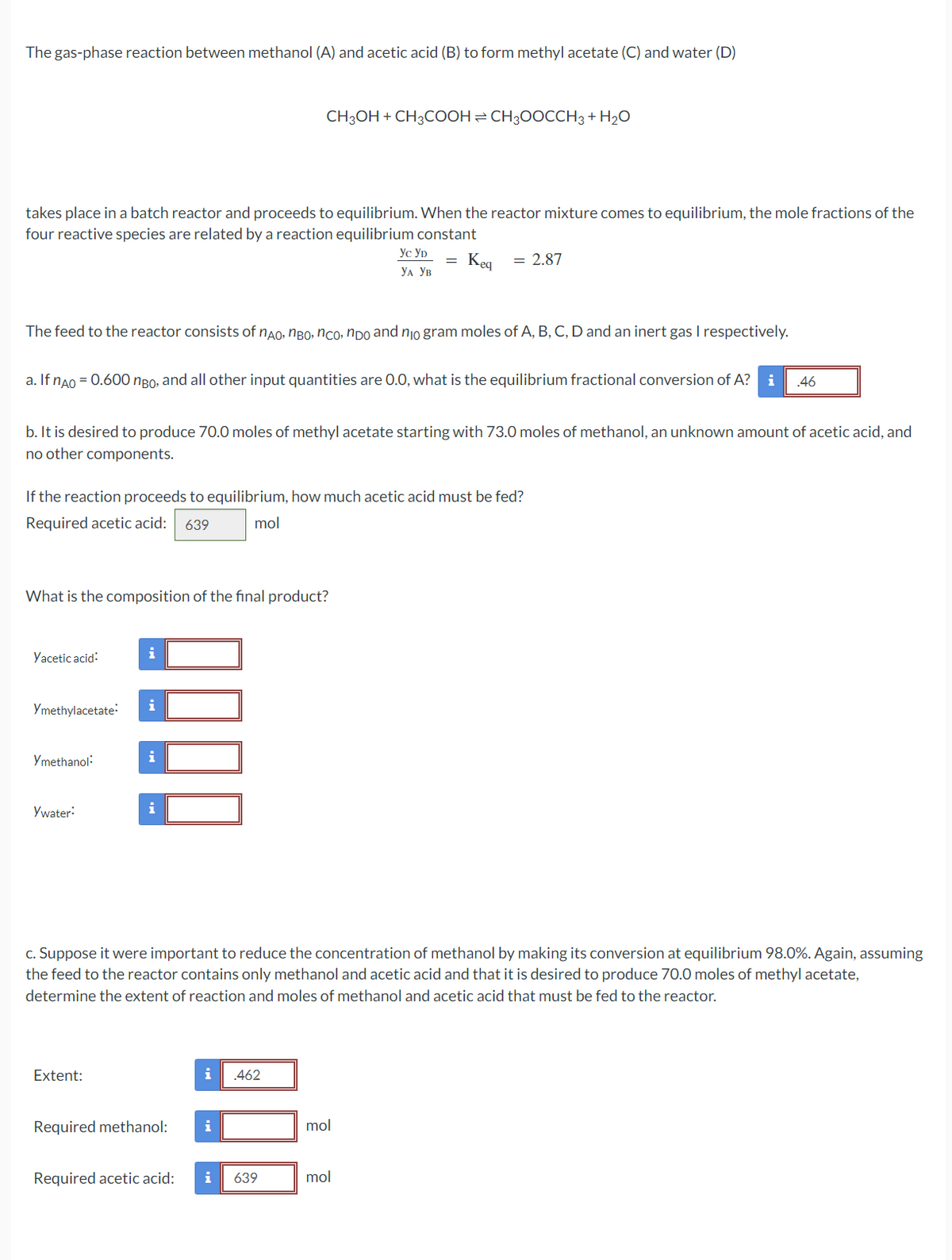
Transcribed Image Text:The gas-phase reaction between methanol (A) and acetic acid (B) to form methyl acetate (C) and water (D)
takes place in a batch reactor and proceeds to equilibrium. When the reactor mixture comes to equilibrium, the mole fractions of the
four reactive species are related by a reaction equilibrium constant
Keq = 2.87
The feed to the reactor consists of não, não, não, não and no gram moles of A, B, C, D and an inert gas I respectively.
a. If nÃO = 0.600 ngo, and all other input quantities are 0.0, what is the equilibrium fractional conversion of A? i .46
b. It is desired to produce 70.0 moles of methyl acetate starting with 73.0 moles of methanol, an unknown amount of acetic acid, and
no other components.
If the reaction proceeds to equilibrium, how much acetic acid must be fed?
Required acetic acid: 639
mol
What is the composition of the final product?
Yacetic acid:
Ymethylacetate:
Ymethanol:
Ywater:
!!!
Extent:
CH3OH + CH3COOH CH3OOCCH3 + H₂O
i
c. Suppose it were important to reduce the concentration of methanol by making its conversion at equilibrium 98.0%. Again, assuming
the feed to the reactor contains only methanol and acetic acid and that it is desired to produce 70.0 moles of methyl acetate,
determine the extent of reaction and moles of methanol and acetic acid that must be fed to the reactor.
Required methanol:
Required acetic acid:
i .462
ус ур
УА УВ
i
i 639
mol
mol
Expert Solution
Trending now
This is a popular solution!
Step by step
Solved in 10 steps

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The