The heat of combustion of 1-pentanol is -3331 kJ/mol. Given the following information, calculate the heat of formation of 1-pentanol. AH° (CO,(g)) = -393-5 kJ/mol AH°; (H,0(1)) = -285.8 kJ/mol kJ/mol
The heat of combustion of 1-pentanol is -3331 kJ/mol. Given the following information, calculate the heat of formation of 1-pentanol. AH° (CO,(g)) = -393-5 kJ/mol AH°; (H,0(1)) = -285.8 kJ/mol kJ/mol
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter10: Fuels, Organic Chemicals, And Polymers
Section10.1: Petroleum
Problem 10.1E: Heptane, C7H16, can be catalytically reformed to make toluene, C6H5CH3, another seven-carbon...
Related questions
Question
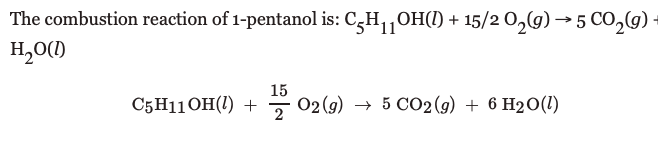
Transcribed Image Text:The combustion reaction of 1-pentanol is: C,H,OH(1) + 15/2 0,(g) → 5 CO,9 -
H,O(1)
15
C5H11OH(1) +
02 (9) → 5 CO2 (9) + 6 H2O(1)

Transcribed Image Text:The heat of combustion of 1-pentanol is -3331 kJ/mol. Given the following information, calculate the
heat of formation of 1-pentanol.
AH°; (CO,(g)) = -393-5 kJ/mol
AH°; (H,0()) = -285.8 kJ/mol
kJ/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning
Expert Answers to Latest Homework Questions
Q: Milaaaaaa.mk
Q: The annual budgeted conversion costs for a lean cell are $554,400 for 3,300 production hours. Each…
Q: Complete and balance the following half-reaction in basic solution. Be sure
to include the proper…
Q: Can you check if my answer is correct
ZT = 720.46Ω∠43.92°
Is(p-p) = 11.1mA∠-43.92°
IL(p-p) =…
Q: Please don't provide handwritten solution ....
Q: Pockets Inc. manufactures cargo pants in the cutting and sewing process. Cargo pants are…
Q: 'red
A certain half-reaction has a standard reduction potential Eed = -0.94 V. An engineer proposes…
Q: Please don't provide handwritten solution ....
Q: A uniform thin rod of length 0.78 m and mass 3.3 kg can rotate in a horizontal plane about a…
Q: The figure shows a rigid structure consisting of a circular hoop of radius R and mass m, and a…
Q: In the figure here, three particles of mass m = 0.022 kg are fastened to three rods of length d =…
Q: Complete the invoice itemization by finding the total amount (in $) for each item, the merchandize…
Q: Can someone please help me to correctly solve all the following parts of this question. Thank you!
Q: Can someone please help me to correctly solve all the following parts of this question. Thank you!
Q: differential equations problem is this correct please help
Q: A1
Q: List 10 key factors of congestion mitigation that may influence freeway mobility performance.
Q: Solve the given initial value problem.
3 4
6
x'(t)=
x(t), x(0)=
4 3
4
x(t)=
Q: Calculate the energy released, in J, when 1.00 kg of
uranium-235 undergoes the following fission…
Q: What are the major types of data that are needed for freeway congestion management? Emphasize on…
Q: assume you have a HashMap class that uses a threshold of 0.75 (75%),regardless of the collision…