The industrial alcohol is fed to an intermediate level of a distillation column. Benzene-rich stream is also fed to the column, commonly known as 'reflux'. The vapour stream (overhead product) leaves the top of the column is a three-component mixture (azeotropic mixture), consisting of benzene, ethanol and water while, the liquid product leaves the bottom column as pure ethanol. The overhead vapour product passes through a condenser and is completely condensed to liquid. However, this liquid is actually having two separate immiscible phases, which are then separated in a decanter into an upper layer (benzene-rich) where this stream is then mixed with pure benzene as make-up for losses. This mixture is fed to the top of column, as above mentioned. The lower layer, rich in ethanol and water is sent to another unit for benzene recovery. Table 1 shows the component weight analysis for each stream. Feed Component Ethanol (E) 95.0 5.0 Water (W) Benzene (B) Table 1: Analysis of % w/w Bottoms Overhead Upper Layer 16.3 product 100.0 vapour 18.5 7.5 74 2.7 81.0 Lower Make-up layer 39.8 54.3 5.9 100.00 With the aid of a completely-labelled flowchart, for a feed rate to the process of 1200 kg/h, determine the production rate of absolute alcohol (kg/h), percentage recovery of ethanol from feed as pure alcohol, flow rate (kg/h) of make-up benzene (pure benzene) and flow rates of upper and lower layers in the decanter (kg/h).
The industrial alcohol is fed to an intermediate level of a distillation column. Benzene-rich stream is also fed to the column, commonly known as 'reflux'. The vapour stream (overhead product) leaves the top of the column is a three-component mixture (azeotropic mixture), consisting of benzene, ethanol and water while, the liquid product leaves the bottom column as pure ethanol. The overhead vapour product passes through a condenser and is completely condensed to liquid. However, this liquid is actually having two separate immiscible phases, which are then separated in a decanter into an upper layer (benzene-rich) where this stream is then mixed with pure benzene as make-up for losses. This mixture is fed to the top of column, as above mentioned. The lower layer, rich in ethanol and water is sent to another unit for benzene recovery. Table 1 shows the component weight analysis for each stream. Feed Component Ethanol (E) 95.0 5.0 Water (W) Benzene (B) Table 1: Analysis of % w/w Bottoms Overhead Upper Layer 16.3 product 100.0 vapour 18.5 7.5 74 2.7 81.0 Lower Make-up layer 39.8 54.3 5.9 100.00 With the aid of a completely-labelled flowchart, for a feed rate to the process of 1200 kg/h, determine the production rate of absolute alcohol (kg/h), percentage recovery of ethanol from feed as pure alcohol, flow rate (kg/h) of make-up benzene (pure benzene) and flow rates of upper and lower layers in the decanter (kg/h).
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
i really need help to calculate this i already draw the flowchart please help ?
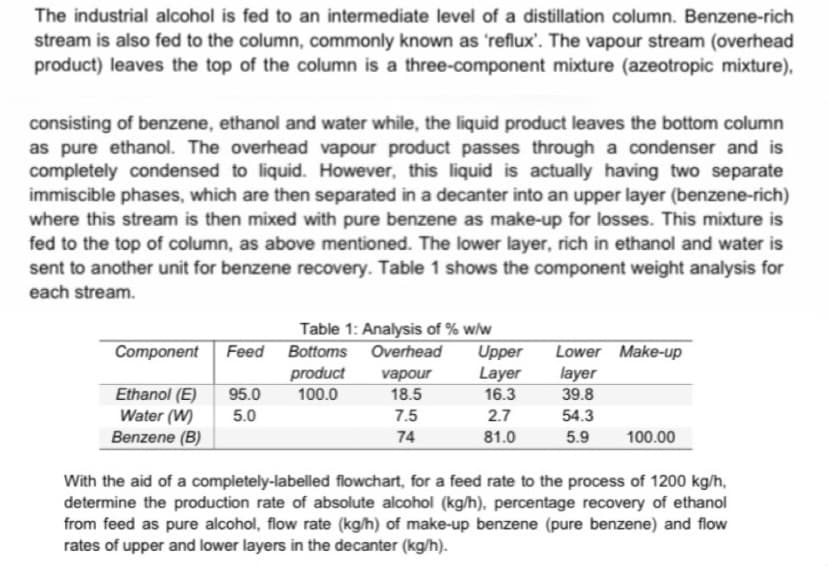
Transcribed Image Text:The industrial alcohol is fed to an intermediate level of a distillation column. Benzene-rich
stream is also fed to the column, commonly known as 'reflux'. The vapour stream (overhead
product) leaves the top of the column is a three-component mixture (azeotropic mixture),
consisting of benzene, ethanol and water while, the liquid product leaves the bottom column
as pure ethanol. The overhead vapour product passes through a condenser and is
completely condensed to liquid. However, this liquid is actually having two separate
immiscible phases, which are then separated in a decanter into an upper layer (benzene-rich)
where this stream is then mixed with pure benzene as make-up for losses. This mixture is
fed to the top of column, as above mentioned. The lower layer, rich in ethanol and water is
sent to another unit for benzene recovery. Table 1 shows the component weight analysis for
each stream.
Table 1: Analysis of % w/w
Upper
Layer
Component
Feed Bottoms Overhead
Lower Make-up
layer
product
100.0
vapour
Ethanol (E)
Water (W)
Benzene (B)
95.0
18.5
16.3
39.8
5.0
7.5
2.7
54.3
74
81.0
5.9
100.00
With the aid of a completely-labelled flowchart, for a feed rate to the process of 1200 kg/h,
determine the production rate of absolute alcohol (kg/h). percentage recovery of ethanol
from feed as pure alcohol, flow rate (kg/h) of make-up benzene (pure benzene) and flow
rates of upper and lower layers in the decanter (kg/h).
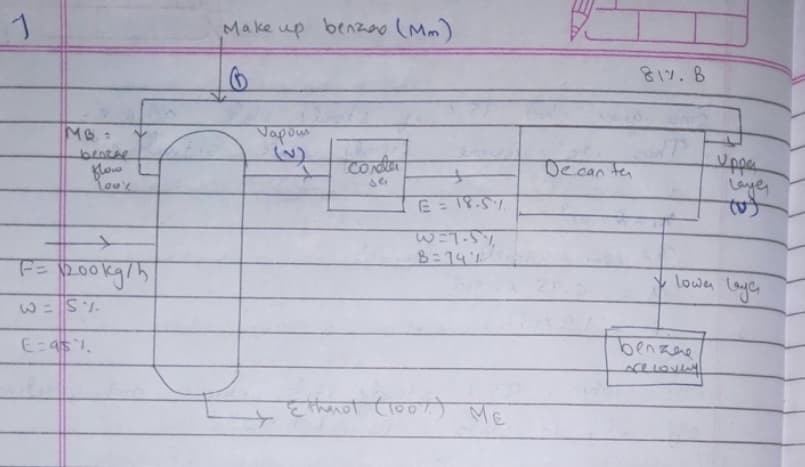
Transcribed Image Text:Make up benzeo (Mm)
817.8
Vapom
(v)
Upper
laye,
Conter
Decan ten
benRne
Blow
se
%3D
7.5.31=
8:14
F=200kg/h
Lown Leye
1. S= 3
benzae
ME
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 1 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The