The isomerization of methyl isocyanide, CH3NC - CH3CN, follows first-order kinetics. The half-lives were found to be 161 min at 199°C and 12.5 min at 230°C. Calculate the activation energy for this reaction. Multiple Choice 124 kJ/mol 78.2 kJ/mol 31.4 kJ/mol 163 KJ/mol
The isomerization of methyl isocyanide, CH3NC - CH3CN, follows first-order kinetics. The half-lives were found to be 161 min at 199°C and 12.5 min at 230°C. Calculate the activation energy for this reaction. Multiple Choice 124 kJ/mol 78.2 kJ/mol 31.4 kJ/mol 163 KJ/mol
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter11: Chemical Kinetics: Rates Of Reactions
Section: Chapter Questions
Problem 41QRT
Related questions
Question
5. Please write clearly

Transcribed Image Text:Multiple Cholce
124 kJ/mol
78.2 kJ/mol
31.4 kJ/mol
163 kJ/mol
6.17 x 10-3 kJ/mol
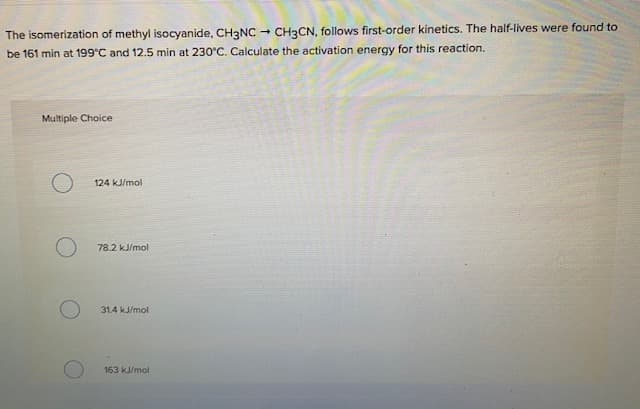
Transcribed Image Text:The isomerization of methyl isocyanide, CH3NC
- CH3CN, follows first-order kinetics. The half-lives were found to
be 161 min at 199°C and 12.5 min at 230°C. Calculate the activation energy for this reaction.
Multiple Choice
124 kJ/mol
78.2 kJ/mol
31.4 kJ/mol
163 kJ/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning