The isotope of plutonium "Pu is used to make thermoelectric power sources for spacecraft. Suppose that a space probe was launched in 2012 with 3.0 238 nd ge Part 1 of 2
The isotope of plutonium "Pu is used to make thermoelectric power sources for spacecraft. Suppose that a space probe was launched in 2012 with 3.0 238 nd ge Part 1 of 2
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter19: Nuclear Chemistry
Section: Chapter Questions
Problem 48AP
Related questions
Question
help
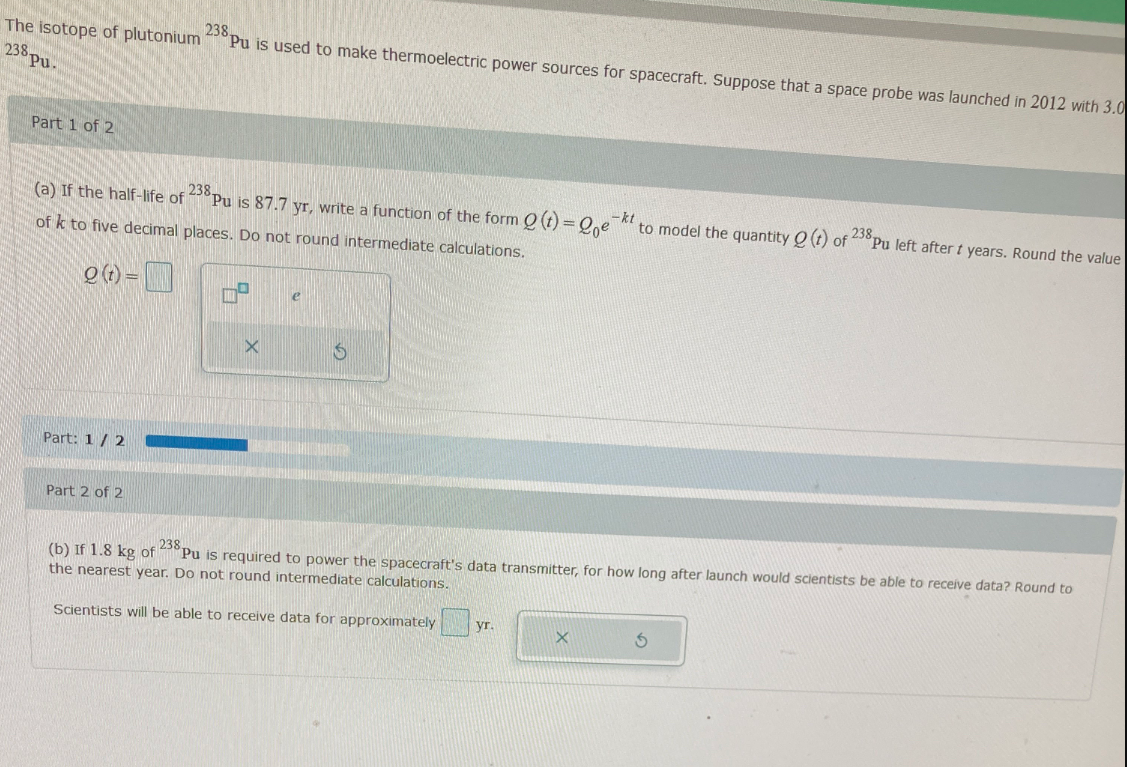
Transcribed Image Text:238.
"Pu is used to make thermoelectric power sources for spacecraft. Suppose that a space probe was launched in 2012 with 3.0
The isotope of plutonium
Part 1 of 2
238.
Pu is 87.7 yr, write a function of the form O (t) = 0,e
(a) If the half-life of
–kt
to model the quantity O (t) of 238
Pu left after t years. Round the value
of k to five decimal places. Do not round intermediate calculations.
O (1) =
e
Part: 1/ 2
Part 2 of 2
238
Pu is required to power the spacecraft's data transmitter, for how long after launch would scientists be able to receive data? Round to
(b) If 1.8 kg of
the nearest year. Do not round intermediate calculations,
Scientists will be able to receive data for approximately
yr.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning