The Ksp of Mg(OH)2 is 5.61 x 10-12, If you tried to dissolve 24.0 mg of Mg(OH)2 in 250 mL of wąter and then filtered the solution and dried the remaining solid, what would you predict to be the mass of the undissolved solid? O 3.34 mg O 0.77 mg 4.05 mg 1.63 mg
The Ksp of Mg(OH)2 is 5.61 x 10-12, If you tried to dissolve 24.0 mg of Mg(OH)2 in 250 mL of wąter and then filtered the solution and dried the remaining solid, what would you predict to be the mass of the undissolved solid? O 3.34 mg O 0.77 mg 4.05 mg 1.63 mg
Chapter10: Potentiometry And Redox Titrations
Section: Chapter Questions
Problem 8P
Related questions
Question
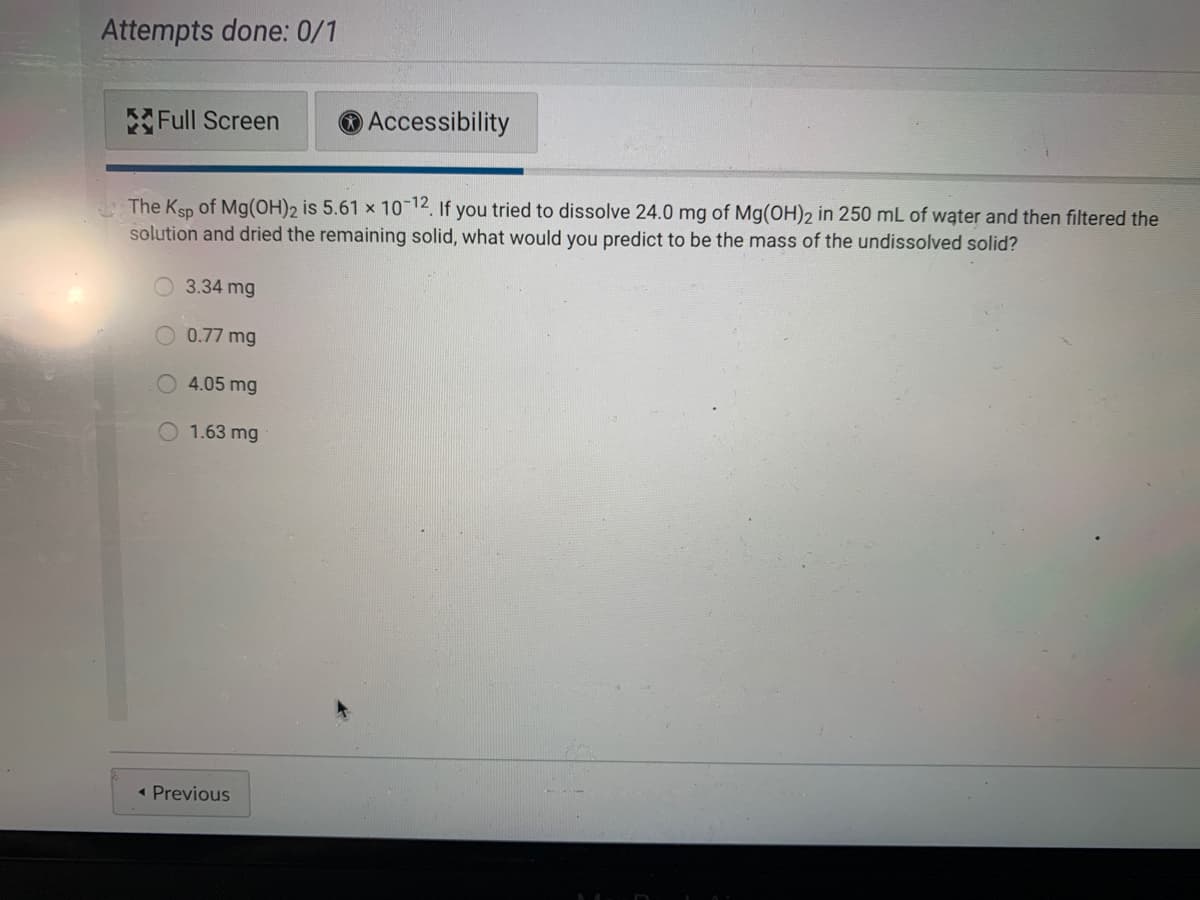
Transcribed Image Text:Attempts done: 0/1
Full Screen
O Accessibility
The Ksp of Mg(OH)2 is 5.61 x 10 12, If you tried to dissolve 24.0 mg of Mg(OH)2 in 250 mL of water and then filtered the
solution and dried the remaining solid, what would you predict to be the mass of the undissolved solid?
O 3.34 mg
0.77 mg
4.05 mg
1.63 mg
« Previous
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



