The [Mg2+] in a saturated solution of Mg3(PO4)2 is 1.18 x 10-5 M. Calculate the Ksp for Mg3(PO4)2- O 9.41 x 10-28 O 6.00 x 10-21 O 5.65 x 10-27 O 1.02 x 10-25
Q: Molecule Shapes Simulation Go to the PhET simulation site and open the following:…
A:
Q: The concentration of Na+ in seawater, 0.481 M, is much higher than in the cytosol, the fluid inside…
A: Given,Concentration of Na+ in seawater = 0.481 MConcentration of cytosol = 12 mMVolume of seawater…
Q: V Show that (35) = Cp/aTV to calculate the pressure in bar. Hint: use the equation sheet to find a…
A: This is a simple problem for application of thermodynamic equation of states and relations. There is…
Q: 3. Provide the missing reagents from the synthesis below. More than one step may be required in a…
A: Organic synthesis is the procedure of following some specific steps, routes and strategies to form…
Q: Write electrochemical cell notation. Write the cell notation for an electrochemical cell consisting…
A: There are two type of cells Electrolytic cell Galvanic cell Electrolytic cell are the cell that…
Q: H₂C H. مال (EtO)P. ë OEt ?
A: Given reaction is witting reaction. Aldehydes or ketones on reaction with phosphorus ylide gives…
Q: A. 0.080 M HNO₂ B. 0.30 M NH 3 C. 1.5x10-8 M KOH D. 4.1x10-5 M HBr
A:
Q: om the reactions shown below, select all the propagation reactions. for for A B H X Br: CI-CI: G H X…
A: Propagation reaction means the reaction where the new radical generated by the reaction of a neutral…
Q: Identify the conjugate base for each acid. conjugate base of H₂CO3: conjugate base of H₂PO4:…
A: A conjugate base contains one less H atom and one more( - )charge than the acid that formed it.
Q: This is an isomer of C4H10O, and it’s not an alcohol. What could the structure be, and what are the…
A: Given that, the molecular formula of an unknown compound is C4H10O. It is not an alcohol. We have to…
Q: A critical reaction in the production of energy to do work or drive chemical reactions in biological…
A: we have to calculate the free energy change for the given reaction
Q: For each system listed in the first column of the table below, decide (if possible) whether the…
A: Answer: In the given questions, processes applied are mentioned to us and we have to find out the…
Q: How many moles of atoms are in 74.0 g of phosphorus?
A:
Q: Which of the following exhibits dipole-dipole forces as its strongest intermolecular force? O NH3…
A: For dipole dipole interaction electronegativity difference is required
Q: Name the following compound: Br
A: we have to name the given compound
Q: 5.8 Use the Gibbs energies of formation in the Resource sec- tion to decide which of the following…
A: Hi there! as there are multiple sub-parts posted. we are solving first three subparts. If you need…
Q: A compound contains 85.7% C and 14.3% H by mass. What is the empirical formula of the compound?
A: The empirical formula, the simplest whole number ratio that defines constituent elements.
Q: 1 2 3 4 2NO₂(g) = N₂O(g) + heat Stresses Applied to an Equilibrium System 5 6 increase temperature 7…
A: According to Le Chatelier's principle, when any stress is applied to a equilibrium condition then…
Q: Write an acitivity series for the generic metals, A, B, C, D if the following observations were…
A:
Q: A 1.00-L container was evacuated, and 0.01 mol of NO and 0.01 mol of Br2 were placed in the…
A: Answer: Ideal gas equation will be used to solve this question which is shown below: P=nRTV Here:…
Q: A chemist prepares a solution of barium acetate BaCH3CO22 by measuring out 230. μmol of barium…
A: Volume of solution = 500 ml = 0.500 L Number of moles = 230 μmol = 0.230 mmol Here we have to find…
Q: Write electron configurations for the following ions of main group elements: (a) N3−, (b) Ba2+,…
A: we have to write the electron configuration of the given ions (a) N3− (b) Ba2+ (c) Be2+
Q: Butyric acid ( C3H7COOH) is a weak acid . What is the pH of a 0.0250 M solution of this acid. Ka =…
A:
Q: Problem 6.10. A water molecule can vibrate in various ways, but the easiest type of vibration to…
A:
Q: Calculate the heat capacity at constant pressure of methane for all its contribution and the total…
A:
Q: Balance the reaction between PbO2 and Fe²+ to form Pb2+ and Fe³+ in acidic solution. When you have…
A: Above reaction after balanced water appear as product and co-efficient is 2. This reaction involved…
Q: Determine the enthalpy for the following reaction, given the enthalpies of formation in the table.…
A:
Q: Devise reasonable synthesis of the target molecules shown below. H Br steps steps но sx
A: Protection of aldehyde with ethylene glycol gives cyclic acetal. Bromo aryl is then converted to…
Q: Balance the reaction between Al³+ and I₂ to form Al and 103 in acidic solution. When you have…
A:
Q: The following chemical equation is balanced. Ca(NO3)2 + Na₂CO3 CaCO3 + NaNO3 O True False
A:
Q: Calculate the Ksp for Li3PO4 if at equilibrium the concentration of PO43- ion is 3.30 x 10-3 M
A: We have to calculate the Ksp for Lithium phosphate
Q: If a person took a dive into liquid nitrogen, what would most likely cause this person to lose their…
A: Aside from being exceedingly cold, liquid nitrogen is also inert, colourless, odourless,…
Q: For a particular reaction, AH = -111.4 kJ and AS = -25.0 J/K. Calculate AG for this reaction at 298…
A:
Q: any drivers report low tire pressure at the start of winter when the temperature drops. If the…
A:
Q: Be sure to answer all parts. The active agent in many hair bleaches is hydrogen peroxide. The amount…
A: Here we are required to find the Moles of hydrogen peroxide present in the sample of bleach.
Q: For eac em listed in the first column of the table below, decide (if possible) whether the change…
A: Here we have to predict whether entropy increase or decrease in the following processes .
Q: What is the conjugate acid of acetamide and its pka value?
A: Acetamide is CH3CONH2. We need to find the conjugate acid of acetamide and it's pKa values.
Q: Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free…
A:
Q: Using the table of ionization constants, calculate the overall equilibrium constant, Koverall, for…
A:
Q: What is the theory of the first law of thermodynamics?
A: According to the first law of thermodynamics, the energy of the universe remains constant. For a…
Q: If the same amount of fuel (methane, ethane, and propane) and oxygen (excess) is subjected to…
A:
Q: Which of the following is true about solution? A solute is a minor component of a solution. All…
A: The solution is defined as the homogenous mixture of atleast two or more than two substance. Every…
Q: A chemist is studying the following equilibirum, which has the given equilibrium constant at a…
A: Given in following question a equilibrium reaction N2 +3H2 =2NH3 ko=1 *10^-1.…
Q: Rank the elements or compounds in the table below in decreasing order of their boiling points. That…
A:
Q: One common problem with hard-boiled eggs is that their shells break in the water. Hard boiling eggs…
A: Egg is about 90% water and 10% protein. Egg protein is formed of long chains of amino acids which…
Q: % Transmittance 4 100- 50- 0- 4000 OH 3000 broad singlet, 1H ,(d) T- doublet, 2H (c) 3 2000 1500…
A: DBE is double bond equivalent. This DBE formula gives us a idea about number of double bond or…
Q: What are the values of A-D that balance the following equation? (A)H2S+ (B)SO2 produces (C)Cs +…
A:
Q: The gas in a 250.0 mL piston experiences a change in pressure from 1.00 atm to 4.40 atm. What is the…
A: Boyle's law: According to Boyle’s law, the absolute pressure exerted by a given mass of an ideal…
Q: In the combustion of C5H8O2 what is the value of the slope expected for the following graphs: a. A…
A: Answer: Combustion reaction of acetyl acetone is shown below: C5H8O2(l)+6O2(g)→5CO2(g)+4H2O
Q: Calculate the solubility product of the following compound from the solubility data. (a) SrF2…
A:
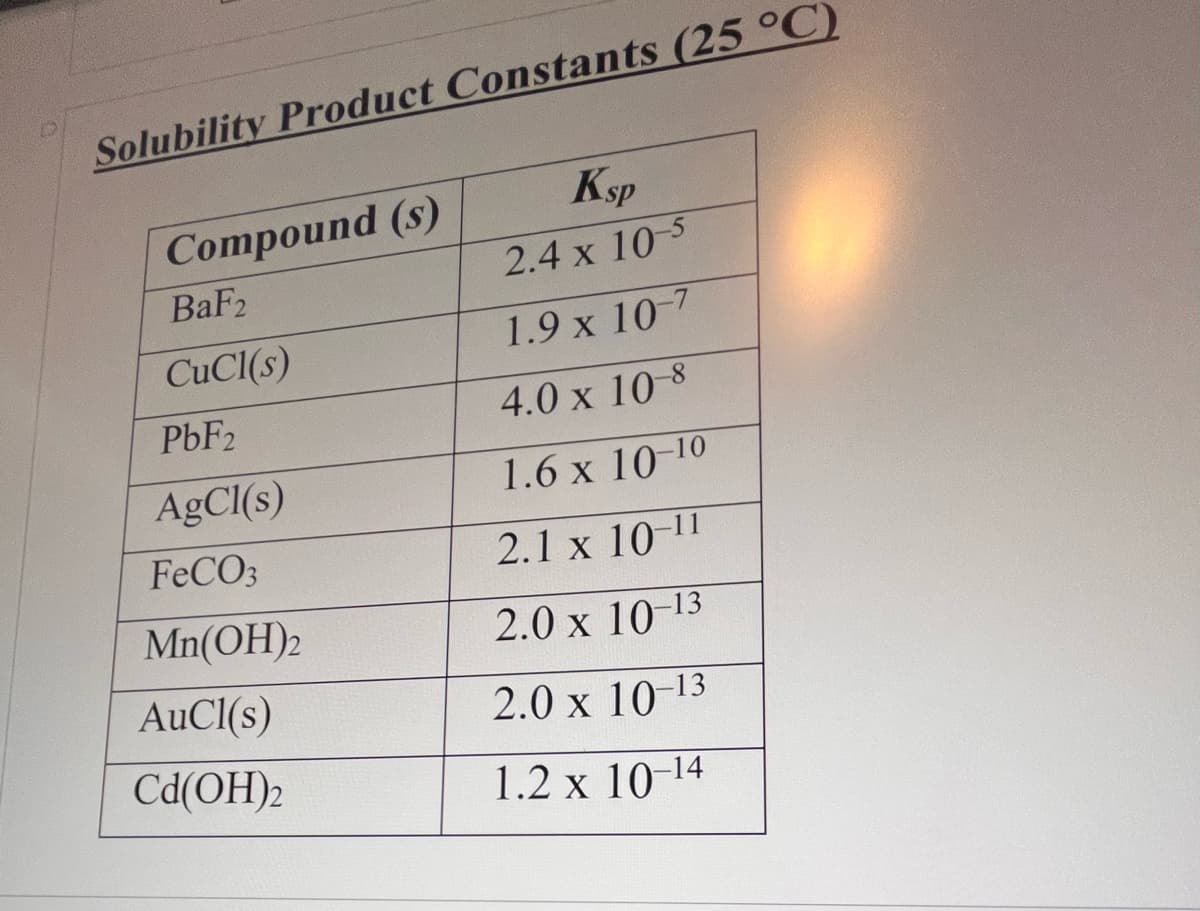
![Question 6
The [Mg2+] in a saturated solution of Mg3(PO4)2 is 1.18 x 10-5 M. Calculate the Ksp for Mg3(PO4)2-
O 9.41 x 10-28
O 6.00 x 10-21
O 5.65 x 10-27
O 1.02 x 10-25](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fbae483e7-f3a4-4a9a-ba35-bb8b04de3604%2Fc86a6e72-df8f-471b-aac8-b77d160cbf82%2Fmb03k3_processed.jpeg&w=3840&q=75)
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

- My question is below: Calculate the solubility (in grams per 100 mL of solution) of magnesium hydroxide in a solution buffered at pH = 12 given that the Ksp for Mg(OH)2 is 2.06x10^-13.common-ion effect: Mg(OH)2 is a sparingly soluble compound, in this case a base, with a solubility product, Ksp of 5.61×10−11 Based on the given value of the Ksp, what is the molar solubility of Mg(OH)2 in 0.140M NaOH?The Ksp value for magnesium arsenate [Mg3(AsO4)2] is 2.00 X 10-20 so if a chemist added 1.19 x 10-2 M of Pb3(AsO4)2(aq) which is a common ion then what would be the concentration of the Arsenate ion AsO43-(aq) in grams per Liter at equilibrium? Question 15 options: 2.44 3.31 4.47 4.23 2.61 3.02 3.78 3.96 2.87 3.57
- Wifey?: What is the Ksp for a compound with generic formula A3X2 if the concentration of X3- ions in a solution saturated with the compound in water is 2.80 x 10-2 M. A. 1.18x10-3 B. 1.51x10-11 C. 4.65x10-7 D. 1.16x10-7 E. 5.81x10-8 Zach Childress: WhatSolubility question again! Calculate the solubility (in g/L) of Aluminum sulfide (Al2S3) in .1 M H2S solution (standrard conditions) {Ksp for Al2S3 is 1.5×10-27}.The concentration of OH– in a saturated solution of Mg(OH)2 is 3.6 × 10–4 M. What is Ksp for Mg(OH)2? Question 32 options: A) 1.3 × 10–7 B) 4.7 × 10–11 C) 1.2 × 10–11 D) 3.6 × 10–4 E) none of these
- A buffer containing acetic acid and sodium acetate has a pH of 5.55. The Ka value for CH3CO2H is 1.80×10^-5. What is the ratio of the concentration of CH3CO2H to CH3CO2^- ?Ksp for AgCl is 1.77E-10. What is the molar solubility of AgCl in a solution of 0.36 M NaCl?Include at least 3 significant figures in your answer.What is the molar solubility of Mg(OH)2 in a basic solution with a pH of 11.00? Ksp for Mg(OH)2 is 5.6x10-12.
- Calcium Phosphate are used to make commercial fertilizer. Its Ksp value is 2.07x10-33 at 25oC. Express answers in 4 significant figures. a. Write the Ksp expression b. What is the concentration of Ca2+ in equilibrium with solid if [PO4 3-] is 5x10-5 M? b. How many moles of PO4 3- are delivered when 275 mL of solution described in sprayed unto a field?What pH is needed to precipitate Ni(OH)2 so completely that the nickel concentration is less than 1.1 x 10-5 M (Ksp = 1.6 x 10-14)? The answer is 9.58.A RbOH solution is titrated four (4) times against potassium hydrogen phthalate (KHP; FW=204.224) samples to the Phenolphthalein endpoint. Using the data below, determine the concentration of the RbOH solution? g of KHP Volume of Base Required 0.5373 g 42.49 mL 0.5856 g 43.88 mL 0.5790 g 48.56 mL 0.5856 g 44.60 mL (Report your answer as "mean +/- std dev") M What is the percent relative standard deviation? % What is the 99% Confidence Interval for the concentration of the solution (population mean)?

