The molar mass of K is O2. Since the stoichiometry has a ration of 4 mol K to mol O2 , K will be the limiting reactant when mass of K is much greater than than times the mass of O2 - 3 much lower than four five 1 comparable to 6 less three 2 more two six
The molar mass of K is O2. Since the stoichiometry has a ration of 4 mol K to mol O2 , K will be the limiting reactant when mass of K is much greater than than times the mass of O2 - 3 much lower than four five 1 comparable to 6 less three 2 more two six
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter5: Stoichiometry
Section: Chapter Questions
Problem 9RQ: Consider the following mixture of SO2(g) and O2(g). If SO2(g) and O2(g) react to form SO3(g), draw a...
Related questions
Question
Consider the following reaction:
4K(s)+O2(g)→2K2O(s)4K(s)+O2(g)→2K2O(s)The molar mass of KK is 39.10 gmol−1gmol−1 and that of O2O2 is 32.00 gmol−1gmol−1.
The limiting reactant is 1.5 g K, 0.38 g O2
I need help with the "explain your reasoning"
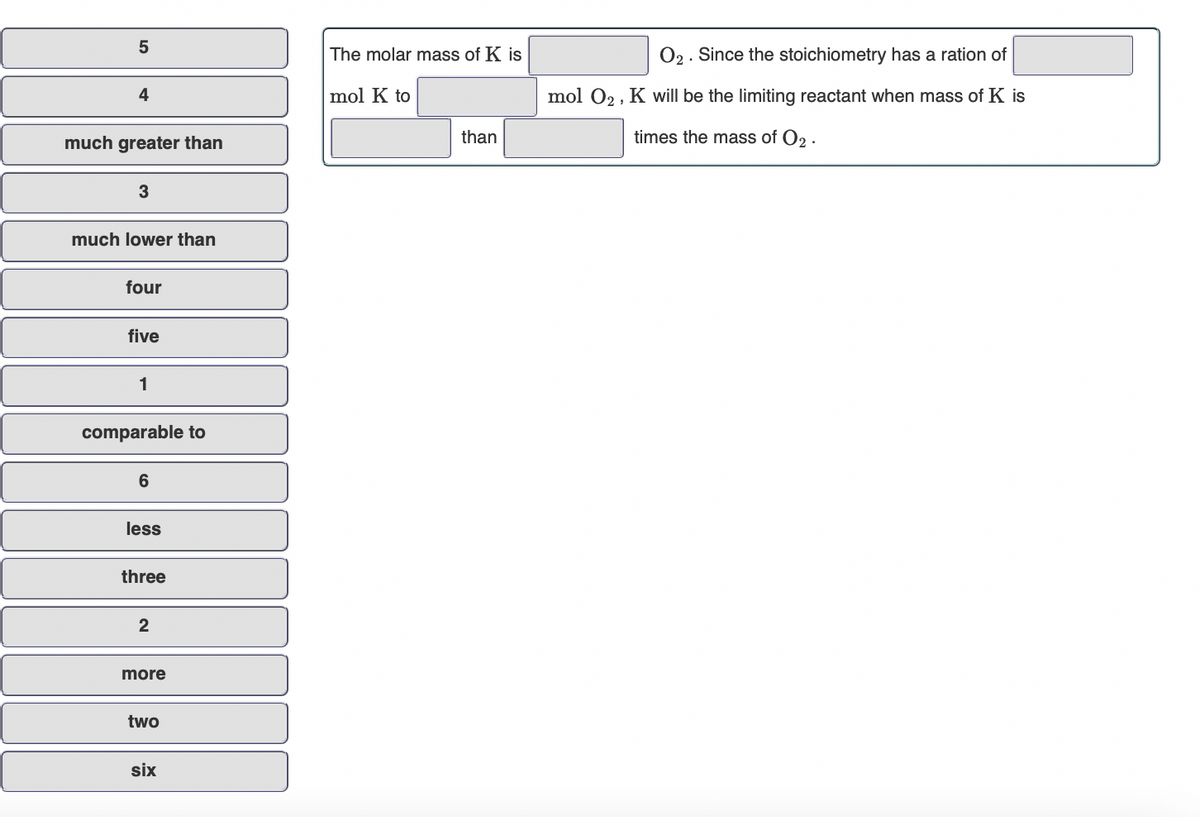
Transcribed Image Text:5
The molar mass of K is
O2. Since the stoichiometry has a ration of
4
mol K to
mol 02, K will be the limiting reactant when mass of K is
much greater than
than
times the mass of O2 .
much lower than
four
five
1
comparable to
6
less
three
2
more
two
six
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co