The molecular diameter of CO is 3.19 x1 08 cm. At 295 Kelvin and a pressure of 100 mm Hg, the number of molecules colliding is 2.23 x 1027 per cubic centimeter per second, the number of bimolecular collisions is 1.12 x 1027 , and the mean free path of the gas is 686 nm. For a pressure of 200 torr and a temperature of 295 Kelvin, determine (a) the number of molecules colliding per cubic centimeter per second, (b) the number of bimolecular collisions, and (c) the mean free path of the gas. How pronounced is the effect of the pressure on the quantities sought? Show your answer in terms of percent difference.
The molecular diameter of CO is 3.19 x1 08 cm. At 295 Kelvin and a pressure of 100 mm Hg, the number of molecules colliding is 2.23 x 1027 per cubic centimeter per second, the number of bimolecular collisions is 1.12 x 1027 , and the mean free path of the gas is 686 nm. For a pressure of 200 torr and a temperature of 295 Kelvin, determine (a) the number of molecules colliding per cubic centimeter per second, (b) the number of bimolecular collisions, and (c) the mean free path of the gas. How pronounced is the effect of the pressure on the quantities sought? Show your answer in terms of percent difference.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter8: Properties Of Gases
Section: Chapter Questions
Problem 119QRT
Related questions
Question
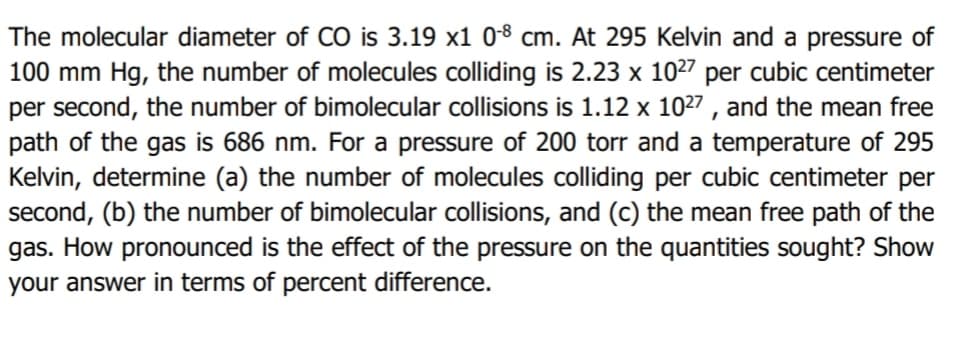
Transcribed Image Text:The molecular diameter of CÓ is 3.19 x1 08 cm. At 295 Kelvin and a pressure of
100 mm Hg, the number of molecules colliding is 2.23 x 1027 per cubic centimeter
per second, the number of bimolecular collisions is 1.12 x 1027 , and the mean free
path of the gas is 686 nm. For a pressure of 200 torr and a temperature of 295
Kelvin, determine (a) the number of molecules colliding per cubic centimeter per
second, (b) the number of bimolecular collisions, and (c) the mean free path of the
gas. How pronounced is the effect of the pressure on the quantities sought? Show
your answer in terms of percent difference.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 4 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning