The Nernst-Planck equation (shown below) describes the motion of a charged chemical species in a fluid. dC; J; = - D; z,FC; dv dx RT dx' What are the units for the ion flux J, where: zis the valence state of the ion (unitless) C is the concentration (mol/m³) Fis the Faraday constant (Coulomb/mol) Ris the ideal gas constant (kg m²/(s² mol °K)) Tis the temperature (K) dci/dx is the concentration gradient (mol/m*) dV/dx is the electric potential gradient (V/m) D; is the diffusion coefficient (m²/s) Note that Coulomb is a unit of charge and V is volts where 1 V= 1 Joule/Coulomb)
The Nernst-Planck equation (shown below) describes the motion of a charged chemical species in a fluid. dC; J; = - D; z,FC; dv dx RT dx' What are the units for the ion flux J, where: zis the valence state of the ion (unitless) C is the concentration (mol/m³) Fis the Faraday constant (Coulomb/mol) Ris the ideal gas constant (kg m²/(s² mol °K)) Tis the temperature (K) dci/dx is the concentration gradient (mol/m*) dV/dx is the electric potential gradient (V/m) D; is the diffusion coefficient (m²/s) Note that Coulomb is a unit of charge and V is volts where 1 V= 1 Joule/Coulomb)
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter25: Voltammetry
Section: Chapter Questions
Problem 25.14QAP
Related questions
Question
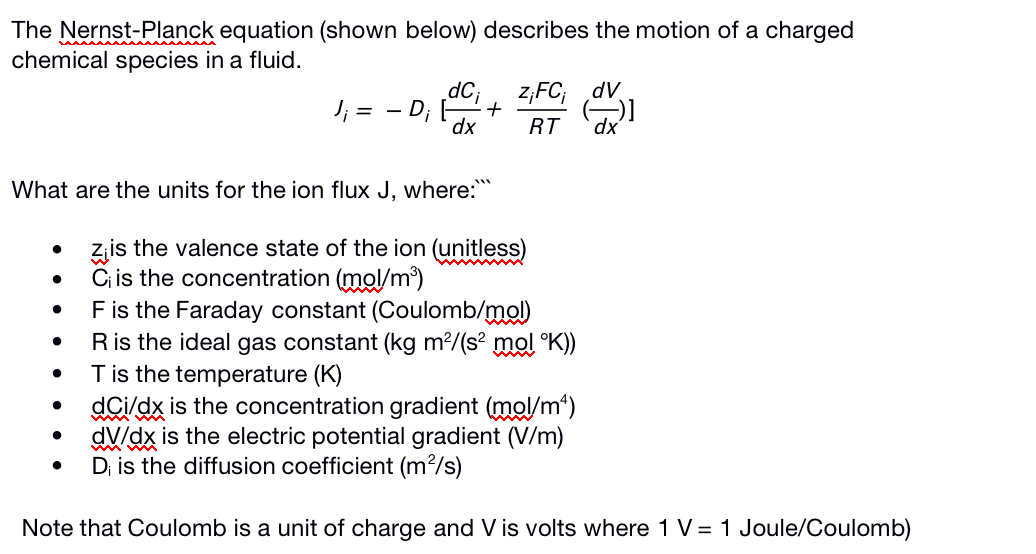
Transcribed Image Text:The Nernst-Planck equation (shown below) describes the motion of a charged
chemical species in a fluid.
dC;
z,FC; dv
J; = - D;
dx
RT
dx'
What are the units for the ion flux J, where:
zis the valence state of the ion (unitless)
C is the concentration (mol/m³)
Fis the Faraday constant (Coulomb/mol)
Ris the ideal gas constant (kg m²/(s² mol °K))
Tis the temperature (K)
dCi/dx is the concentration gradient (mol/m*)
dV/dx is the electric potential gradient (V/m)
D; is the diffusion coefficient (m²/s)
Note that Coulomb is a unit of charge and V is volts where 1 V= 1 Joule/Coulomb)
Expert Solution
Given:

Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
