The-NH, group in aniline is not completely planar with the benzene ring. Specifically, the angle to the benzene ring and the bisector of the amino group is 140° instead of 180°. By comparison, the analogous angle in methylamine is 125°, while in p-nitroaniline it is indeed 180°. H,C-N H HA H 140° 180 125 Aniline Methylamine p-Nitroaniline
The-NH, group in aniline is not completely planar with the benzene ring. Specifically, the angle to the benzene ring and the bisector of the amino group is 140° instead of 180°. By comparison, the analogous angle in methylamine is 125°, while in p-nitroaniline it is indeed 180°. H,C-N H HA H 140° 180 125 Aniline Methylamine p-Nitroaniline
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter27: Amino Acids And Proteins
Section: Chapter Questions
Problem 27.39P: A chemically modified guanidino group is present in cimetidine (Tagamet), a widely prescribed drug...
Related questions
Question
Q.The angle found in p-nitroaniline means that the
1. The nitro group withdraws the lone pair electrons from the amine, primarily via induction, making the N atom sp2 hybridized and hence trigonal planar.
2. The nitro group withdraws the lone pair electrons from the amine, primarily via
resonance, making the N atom sp2 hybridized and hence trigonal planar.
3. The lone pair of the N atom of the NH2 must be in a p orbital to make the system
4. The nitrogen of an amine is usually planar, and aniline and methylamine are exceptions.
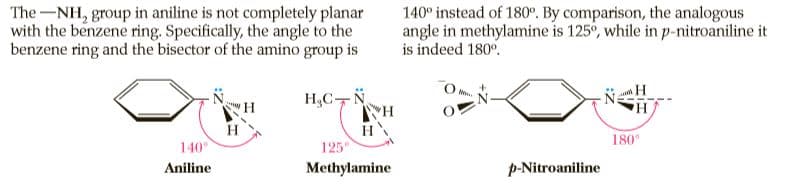
Transcribed Image Text:The-NH, group in aniline is not completely planar
with the benzene ring. Specifically, the angle to the
benzene ring and the bisector of the amino group is
140° instead of 180°. By comparison, the analogous
angle in methylamine is 125°, while in p-nitroaniline it
is indeed 180°.
H,C-N
H
HA
H
140°
180
125
Aniline
Methylamine
p-Nitroaniline
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning