The nitro groups on the benzene ring in the reactant serve two purposes. One is to let you know what atoms in the reactant correspond to what atoms in the product. But what role do the nitro groups play electronically – why would the reaction be much slower if these nitro groups weren’t attached to those benzene carbons? Draw any relevant structures to support your answer
The nitro groups on the benzene ring in the reactant serve two purposes. One is to let you know what atoms in the reactant correspond to what atoms in the product. But what role do the nitro groups play electronically – why would the reaction be much slower if these nitro groups weren’t attached to those benzene carbons? Draw any relevant structures to support your answer
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter3: Composition Of Substances And Solutions
Section: Chapter Questions
Problem 70E: What mass of a 4.00% NaOH solution by mass contains 15.0 g of NaOH?
Related questions
Question
The nitro groups on the benzene ring in the reactant serve two purposes.
One is to let you know what atoms in the reactant correspond to what atoms
in the product. But what role do the nitro groups play electronically – why
would the reaction be much slower if these nitro groups weren’t attached to
those benzene carbons? Draw any relevant structures to support your
answer.
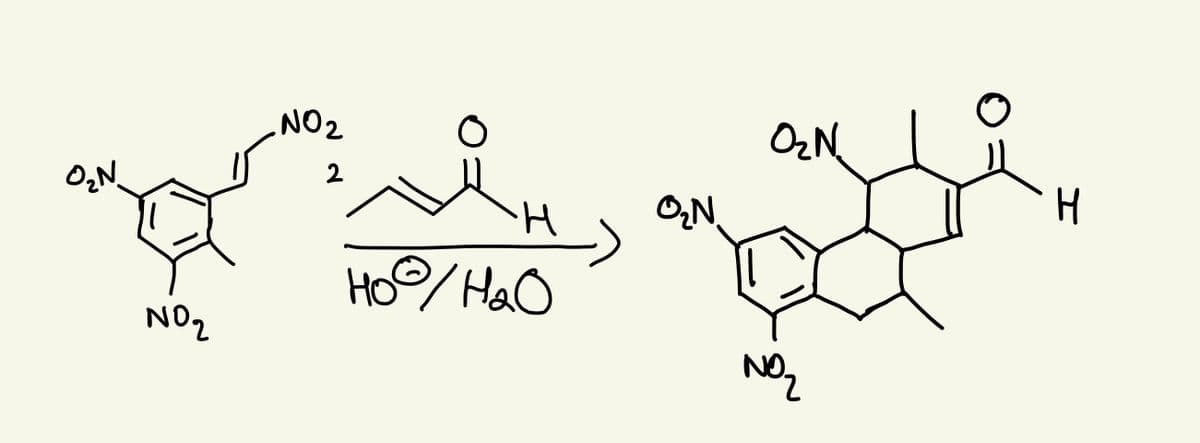
Transcribed Image Text:O₂N.
NO₂
.NO ₂
H
Hoo/H₂O
O₂N.
O₂N.
NO₂
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning