The nonvolatile, nonelectrolyte estrogen (estradiol), C13H2402 (272.40 g/mol), is soluble in diethyl ether CH3CH,OCH2CH3. Calculate the osmotic pressure generated when 13.9 grams of estrogen are dissolved in 220 ml of a diethyl ether solution at 298 K. The molarity of the solution is ( M. The osmotic pressure of the solution is | | atmospheres.
The nonvolatile, nonelectrolyte estrogen (estradiol), C13H2402 (272.40 g/mol), is soluble in diethyl ether CH3CH,OCH2CH3. Calculate the osmotic pressure generated when 13.9 grams of estrogen are dissolved in 220 ml of a diethyl ether solution at 298 K. The molarity of the solution is ( M. The osmotic pressure of the solution is | | atmospheres.
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter13: Solutions And Their Behavior
Section13.4: Colligative Properties
Problem 13.9CYU: A 1.40-g sample of polyethylene, a common plastic, is dissolved in enough organic solvent to give...
Related questions
Question
please explain
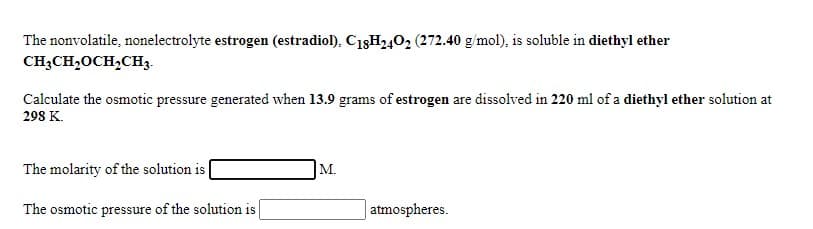
Transcribed Image Text:The nonvolatile, nonelectrolyte estrogen (estradiol), C18H402 (272.40 g/mol), is soluble in diethyl ether
CH;CH,OCH,CH3.
Calculate the osmotic pressure generated when 13.9 grams of estrogen are dissolved in 220 ml of a diethyl ether solution at
298 K.
The molarity of the solution is
M.
The osmotic pressure of the solution is
|atmospheres.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning