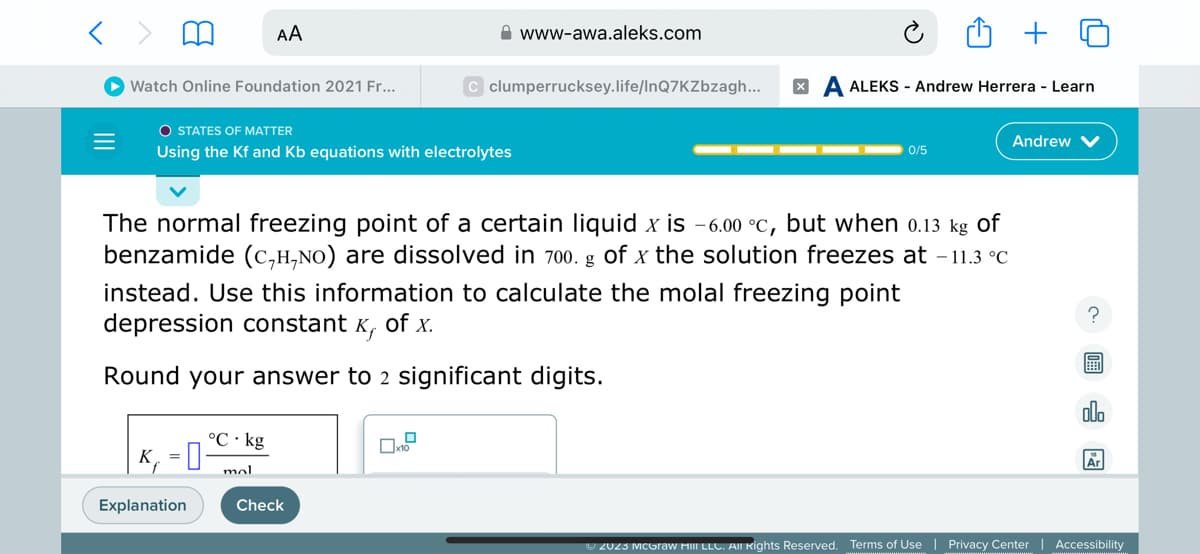
The normal freezing point of a certain liquid x is -6.00 °c, but when 0.13 kg of benzamide (C,H,NO) are dissolved in 700. g of x the solution freezes at -11.3 °C instead. Use this information to calculate the molal freezing point depression constant K, of x. Round your answer to 2 significant digits. °C kg K₁-C-kg = mal
The normal freezing point of a certain liquid x is -6.00 °c, but when 0.13 kg of benzamide (C,H,NO) are dissolved in 700. g of x the solution freezes at -11.3 °C instead. Use this information to calculate the molal freezing point depression constant K, of x. Round your answer to 2 significant digits. °C kg K₁-C-kg = mal
Chapter13: Titrations In Analytical Chemistry
Section: Chapter Questions
Problem 13.10QAP
Related questions
Question
100%
Kf kb,
Transcribed Image Text:< >
=
Watch Online Foundation 2021 Fr...
AA
O STATES OF MATTER
Using the Kf and Kb equations with electrolytes
K₁
Explanation
°C. kg
mal
www-awa.aleks.com
C clumperrucksey.life/InQ7KZbzagh... XA ALEKS - Andrew Herrera - Learn
The normal freezing point of a certain liquid x is -6.00 °c, but when 0.13 kg of
benzamide (C,H,NO) are dissolved in 700. g of x the solution freezes at -11.3 °C
instead. Use this information to calculate the molal freezing point
depression constant x, of x.
Round your answer to 2 significant digits.
Check
0/5
Andrew
B
allo
Ar
Ⓒ2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

