The nuclear binding energy is the energy required to split a nucleus of an atom in its component parts: protons and neutrons, or, collectively, the nucleons. It describes how strongly nucleons are bound to each other. When a high amount of energy is needo
The nuclear binding energy is the energy required to split a nucleus of an atom in its component parts: protons and neutrons, or, collectively, the nucleons. It describes how strongly nucleons are bound to each other. When a high amount of energy is needo
Elements Of Electromagnetics
7th Edition
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Sadiku, Matthew N. O.
ChapterMA: Math Assessment
Section: Chapter Questions
Problem 1.1MA
Related questions
Question
Java
Transcribed Image Text:Toject
Yourld).
Problem:
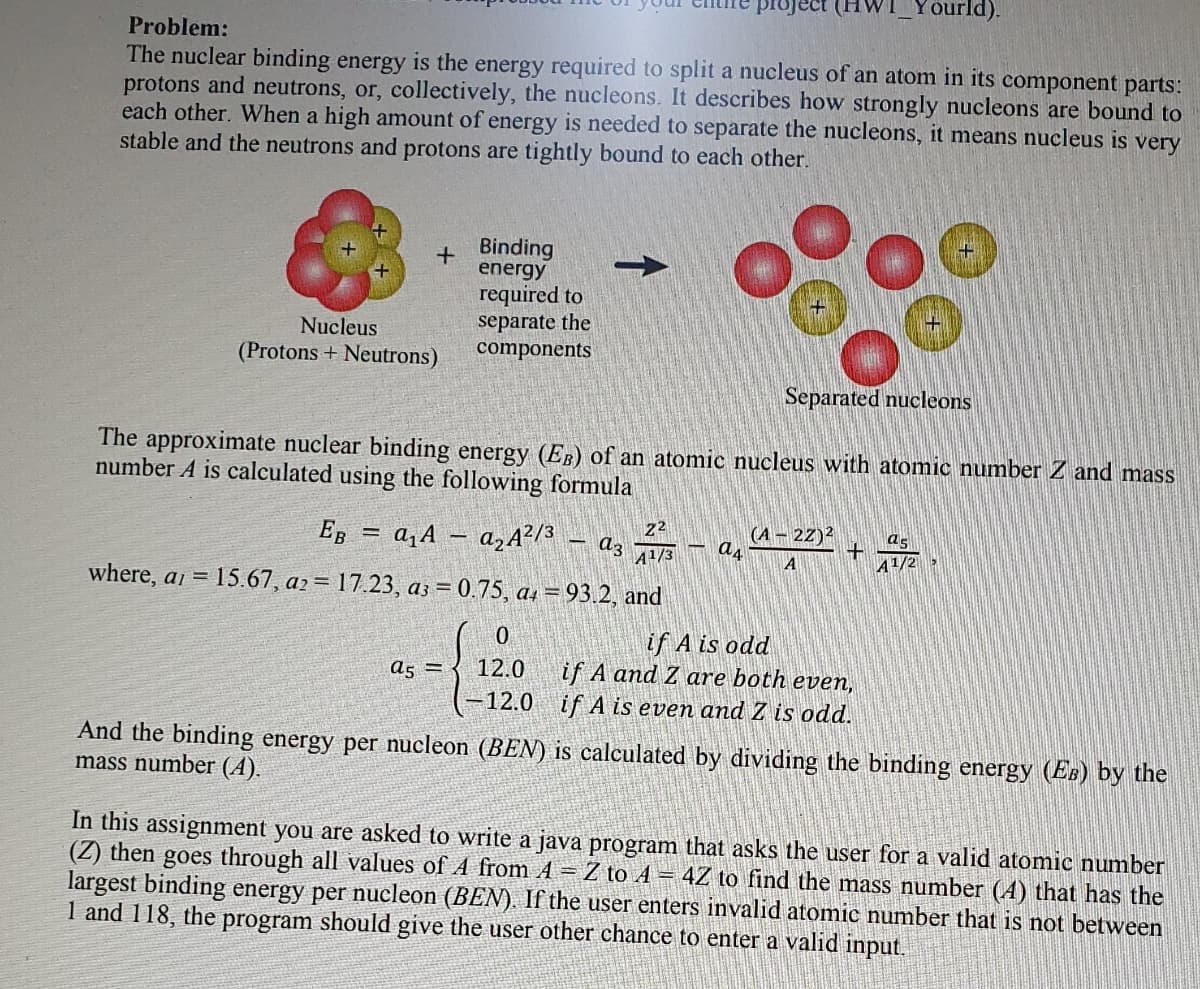
The nuclear binding energy is the energy required to split a nucleus of an atom in its component parts:
protons and neutrons, or, collectively, the nucleons. It describes how strongly nucleons are bound to
each other. When a high amount of energy is needed to separate the nucleons, it means nucleus is very
stable and the neutrons and protons are tightly bound to each other.
+ Binding
energy
required to
separate the
components
Nucleus
(Protons + Neutrons)
Separated nucleons
The approximate nuclear binding energy (EB) of an atomic nucleus with atomic number Z and mass
number A is calculated using the following formula
EB = a,A – azA?/3
2
(А — 22)2
%3D
az
A1/3
A
A1/
where, a = 15.67, a2= 17.23, a3 = 0.75, as = 93.2, and
if A is odd
if A and Z are both even,
if A is even and Z is odd.
a5 =
12.0
-12.0
And the binding energy per nucleon (BEN) is calculated by dividing the binding energy (Es) by the
mass number (A).
In this assignment you are asked to write a java program that asks the user for a valid atomic number
(Z) then goes through all values of A from A = Z to A = 4Z to find the mass number (4) that has the
largest binding energy per nucleon (BEN). If the user enters invalid atomic number that is not between
1 and 118, the program should give the user other chance to enter a valid input.
![run:
Please enter a valid atomic number (Z) [1,118]:> 0
Please enter a valid atomic number (Z) [1,118]:> -4
Please enter a valid atomic number (Z) [1,118]:> 120
Please enter a valid atomic number (Z) (1,118):> 5
Binding
Energy
A
Binding Energy
per Nucleon
5.
6.
-448.996
-226.623
=82.990
-3.778
47.111
-89.799
-37.771
-11.856
-0.472
5.235
6.423
8.
6.
64.228
70.245
55.009
35.952
1.794
-32.682
6.386
12
13
14
4.584
2.766
0.128
-2.179
-4.927
-7.262
-9.869
-12.069
-14.457
15
16
17
-78.825
-123.453
-177.641
-229.307
-289.143
18
19
20
The most stable nucleos has a mass number 10
BUILD SUCCESSFUL (total time: 10 seconds)
Figure 1: Sample run of the program
O H2m456 N](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fbd476f98-9819-4ab6-b65f-61551ccab8fa%2F8b21ff46-781a-49cf-b9d9-10eab744b5bd%2Fd5q3kd8_processed.jpeg&w=3840&q=75)
Transcribed Image Text:run:
Please enter a valid atomic number (Z) [1,118]:> 0
Please enter a valid atomic number (Z) [1,118]:> -4
Please enter a valid atomic number (Z) [1,118]:> 120
Please enter a valid atomic number (Z) (1,118):> 5
Binding
Energy
A
Binding Energy
per Nucleon
5.
6.
-448.996
-226.623
=82.990
-3.778
47.111
-89.799
-37.771
-11.856
-0.472
5.235
6.423
8.
6.
64.228
70.245
55.009
35.952
1.794
-32.682
6.386
12
13
14
4.584
2.766
0.128
-2.179
-4.927
-7.262
-9.869
-12.069
-14.457
15
16
17
-78.825
-123.453
-177.641
-229.307
-289.143
18
19
20
The most stable nucleos has a mass number 10
BUILD SUCCESSFUL (total time: 10 seconds)
Figure 1: Sample run of the program
O H2m456 N
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:
9781118170519
Author:
Norman S. Nise
Publisher:
WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:
9781337093347
Author:
Barry J. Goodno, James M. Gere
Publisher:
Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:
9781118807330
Author:
James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:
WILEY