The nucleophilic aromatic substitution reaction of a halobenzene with a strong base (KN(CH3)2 in NH(CH3)2) involves a benzyne intermediate. Complete the mechanism below by providing the missing curved arrow notation, lone pair electrons, and formal charges. Do not add additional structures or counterions. ici. ci ငေး H i i Add the missing curved arrow notation, lone pair electrons, and formal charges to all atoms.
The nucleophilic aromatic substitution reaction of a halobenzene with a strong base (KN(CH3)2 in NH(CH3)2) involves a benzyne intermediate. Complete the mechanism below by providing the missing curved arrow notation, lone pair electrons, and formal charges. Do not add additional structures or counterions. ici. ci ငေး H i i Add the missing curved arrow notation, lone pair electrons, and formal charges to all atoms.
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter8: Addition Via Carbocation
Section: Chapter Questions
Problem 9E
Related questions
Question
Fix this for me
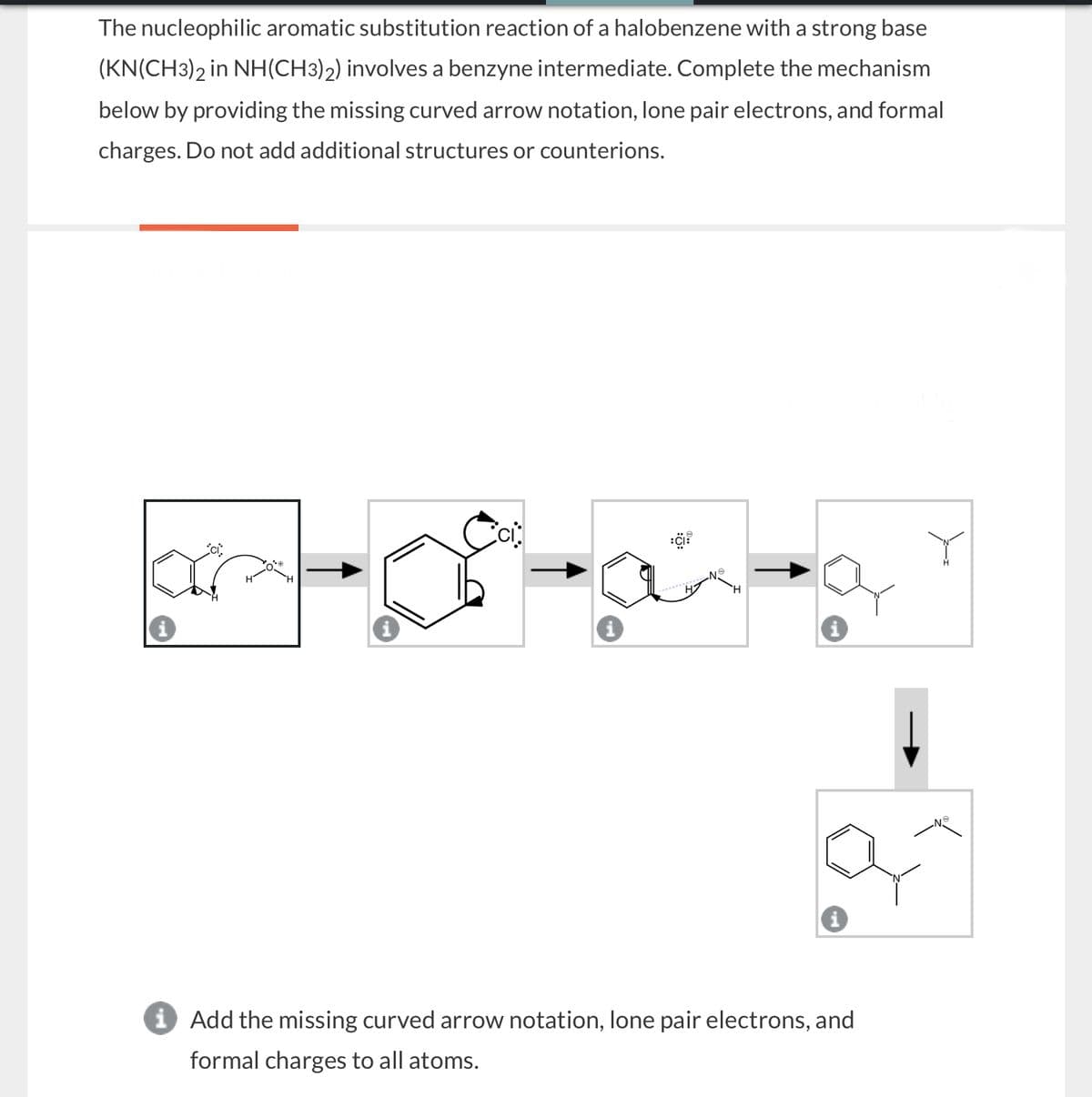
Transcribed Image Text:The nucleophilic aromatic substitution reaction of a halobenzene with a strong base
(KN(CH3)2 in NH(CH3)2) involves a benzyne intermediate. Complete the mechanism
below by providing the missing curved arrow notation, lone pair electrons, and formal
charges. Do not add additional structures or counterions.
ici.
ci
ငေး
H
i
i Add the missing curved arrow notation, lone pair electrons, and
formal charges to all atoms.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning