The osmotic pressure of a 6.60 mL aqueous solution which contains 11.5 mg of insulin is measured to be 5.41 torr at 298 K. What is the 4. molecular weight of insulin? Consider insulin to be a molecular solute. Recall that 760 torr= 1 atmosphere. D. 231 g/mol E. 5,990,000 g/mol A. 5990 g/mol B. 303 g/mol C. 231,000 g/mol
The osmotic pressure of a 6.60 mL aqueous solution which contains 11.5 mg of insulin is measured to be 5.41 torr at 298 K. What is the 4. molecular weight of insulin? Consider insulin to be a molecular solute. Recall that 760 torr= 1 atmosphere. D. 231 g/mol E. 5,990,000 g/mol A. 5990 g/mol B. 303 g/mol C. 231,000 g/mol
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter10: Solutions
Section: Chapter Questions
Problem 62QAP: An aqueous solution made up of 32.47 g of iron(III) chloride in 100.0 mL of solution has a density...
Related questions
Question
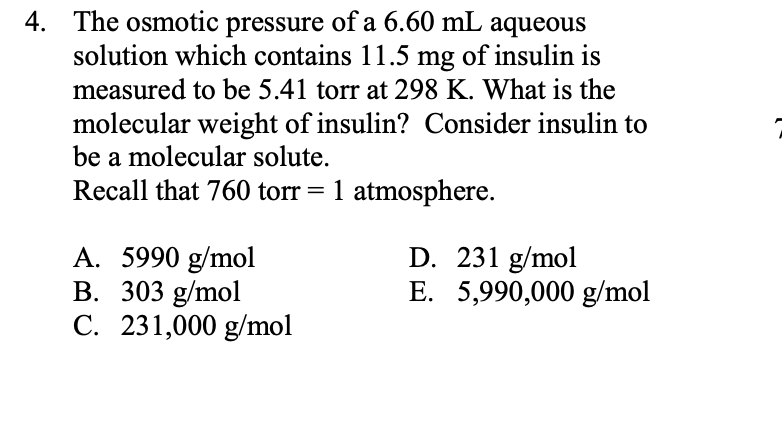
Transcribed Image Text:The osmotic pressure of a 6.60 mL aqueous
solution which contains 11.5 mg of insulin is
measured to be 5.41 torr at 298 K. What is the
4.
molecular weight of insulin? Consider insulin to
be a molecular solute.
Recall that 760 torr= 1 atmosphere.
D. 231 g/mol
E. 5,990,000 g/mol
A. 5990 g/mol
B. 303 g/mol
C. 231,000 g/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 4 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
