The p-V diagram in the figure shows two paths along which a sample of gas can be taken from state a to state b, where V, = 9.0V1. Path 1 requires that energy equal to 20.00p1V1 be transferred to the gas as heat. Path 2 requires that energy equal to 21.50p1V1 be transferred to the gas as heat. What is the ratio p2/p1? a 9. V V1
The p-V diagram in the figure shows two paths along which a sample of gas can be taken from state a to state b, where V, = 9.0V1. Path 1 requires that energy equal to 20.00p1V1 be transferred to the gas as heat. Path 2 requires that energy equal to 21.50p1V1 be transferred to the gas as heat. What is the ratio p2/p1? a 9. V V1
Principles of Physics: A Calculus-Based Text
5th Edition
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter17: Energy In Thermal Processes: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 39P: A 1.00-mol sample of hydrogen gas is heated at constant pressure from 300 K to 420 K. Calculate (a)...
Related questions
Question
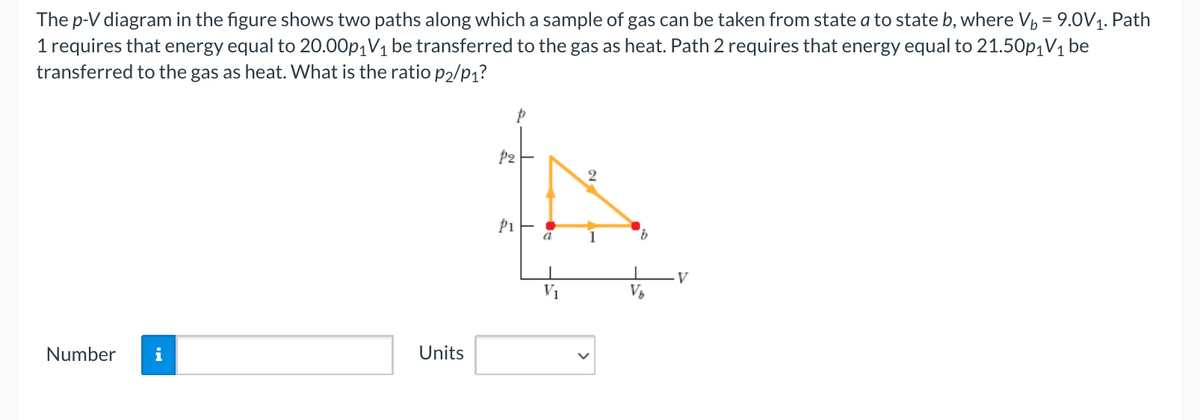
Transcribed Image Text:The p-V diagram in the figure shows two paths along which a sample of gas can be taken from state a to state b, where Vp = 9.0V1. Path
1 requires that energy equal to 20.00p|V1 be transferred to the gas as heat. Path 2 requires that energy equal to 21.50p¡V1 be
transferred to the gas as heat. What is the ratio p2/p1?
P2
2
P1
a
1
V
V1
Number
i
Units
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning