Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter18: Functional Derivatives Of Carboxylic Acids
Section: Chapter Questions
Problem 18.69P
Related questions
Question
12
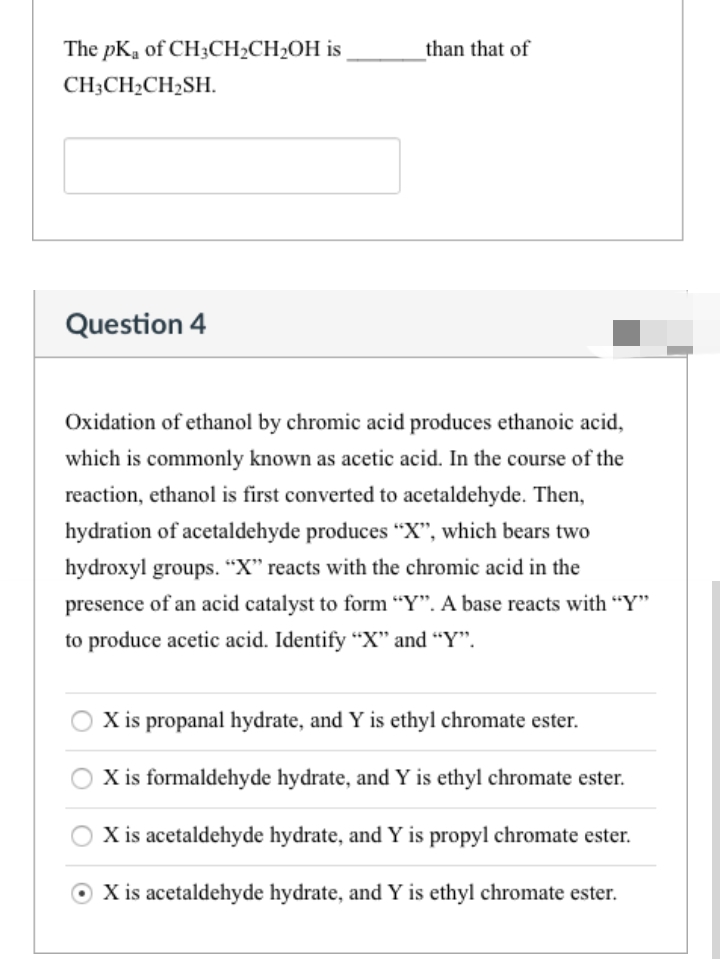
Transcribed Image Text:The pKa of CH3CH2CH2OH is
than that of
CH;CH2CH2SH.
Question 4
Oxidation of ethanol by chromic acid produces ethanoic acid,
which is commonly known as acetic acid. In the course of the
reaction, ethanol is first converted to acetaldehyde. Then,
hydration of acetaldehyde produces "X", which bears two
hydroxyl groups. “X" reacts with the chromic acid in the
presence of an acid catalyst to form “Y". A base reacts with "Y"
to produce acetic acid. Identify “X" and “Y".
X is propanal hydrate, and Y is ethyl chromate ester.
X is formaldehyde hydrate, and Y is ethyl chromate ester.
X is acetaldehyde hydrate, and Y is propyl chromate ester.
X is acetaldehyde hydrate, and Y is ethyl chromate ester.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning
