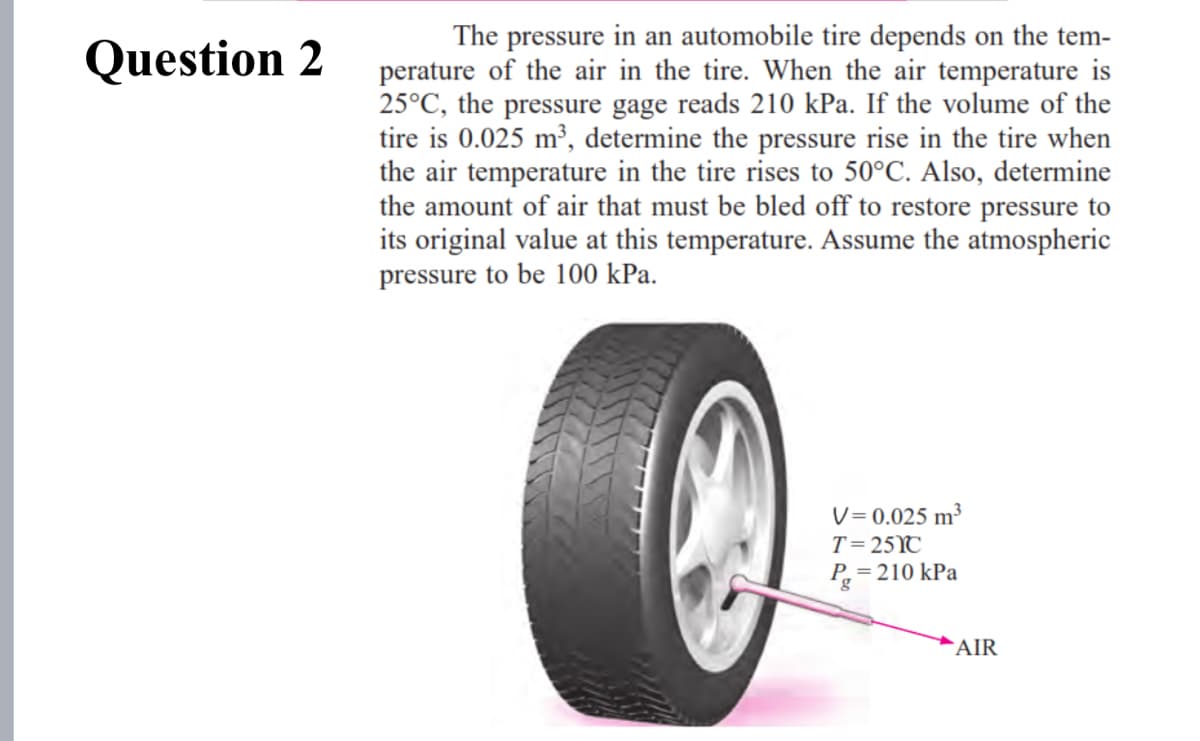
The pressure in an automobile tire depends on the tem- perature of the air in the tire. When the air temperature is 25°C, the pressure gage reads 210 kPa. If the volume of the tire is 0.025 m³, determine the pressure rise in the tire when the air temperature in the tire rises to 50°C. Also, determine the amount of air that must be bled off to restore pressure to its original value at this temperature. Assume the atmospheric pressure to be 100 kPa. V = 0.025 m³ T=25YC P,=210 kPa B, `AIR
Energy transfer
The flow of energy from one region to another region is referred to as energy transfer. Since energy is quantitative; it must be transferred to a body or a material to work or to heat the system.
Molar Specific Heat
Heat capacity is the amount of heat energy absorbed or released by a chemical substance per the change in temperature of that substance. The change in heat is also called enthalpy. The SI unit of heat capacity is Joules per Kelvin, which is (J K-1)
Thermal Properties of Matter
Thermal energy is described as one of the form of heat energy which flows from one body of higher temperature to the other with the lower temperature when these two bodies are placed in contact to each other. Heat is described as the form of energy which is transferred between the two systems or in between the systems and their surrounding by the virtue of difference in temperature. Calorimetry is that branch of science which helps in measuring the changes which are taking place in the heat energy of a given body.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps




