The product in the following reaction is formed as a result of a 1,2-methyl shift from the initially formed 2° carbocation. Which intermediate correctly illustrates that carbocation rearrangement? 5. H,SO, H,O OH2
The product in the following reaction is formed as a result of a 1,2-methyl shift from the initially formed 2° carbocation. Which intermediate correctly illustrates that carbocation rearrangement? 5. H,SO, H,O OH2
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter14: Elimination
Section: Chapter Questions
Problem 46CTQ
Related questions
Question
100%
Orgo! Located in photo.
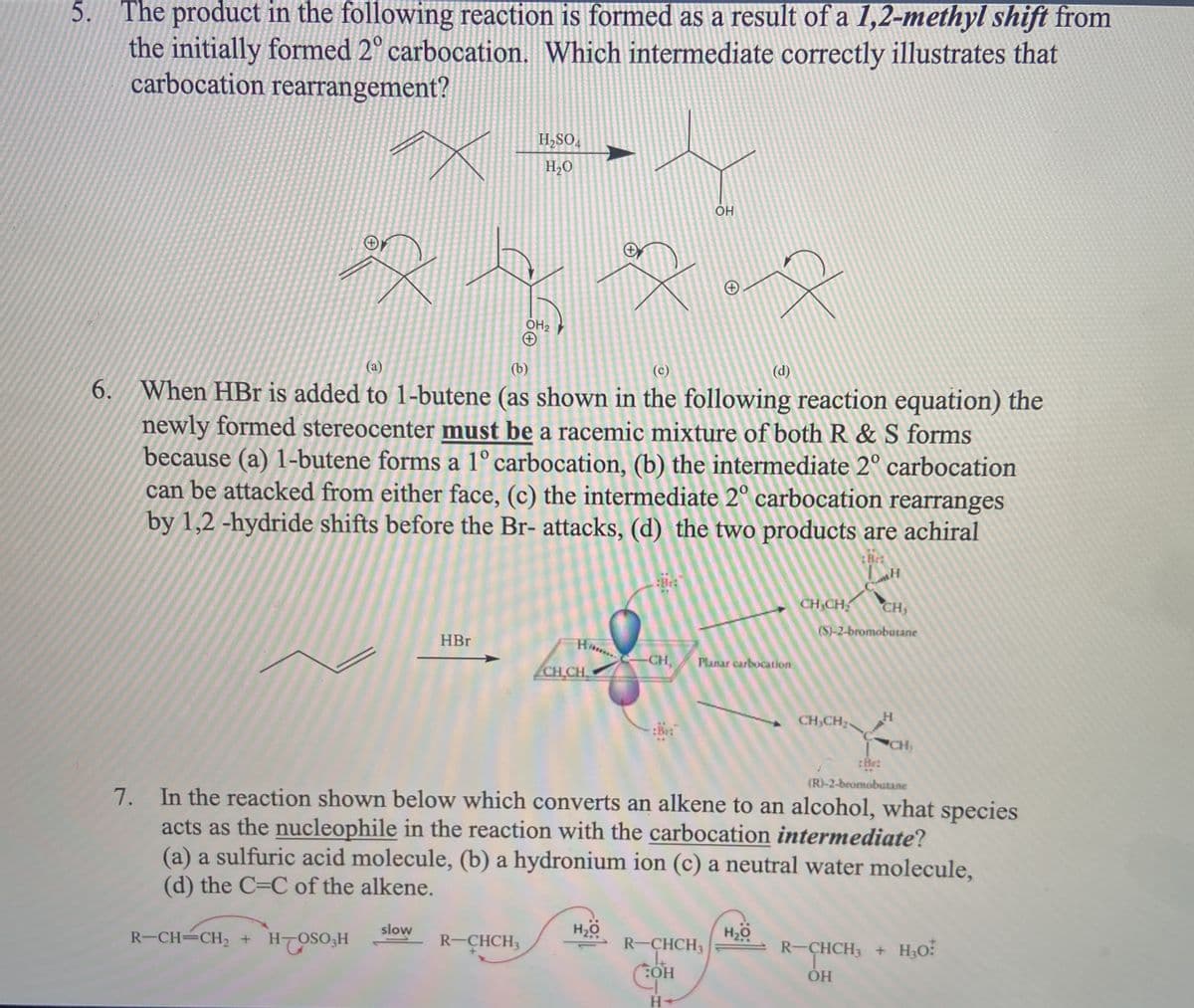
Transcribed Image Text:5.
The product in the following reaction is formed as a result of a 1,2-methyl shift from
the initially formed 2° carbocation. Which intermediate correctly illustrates that
carbocation rearrangement?
H,SO,
H,O
OH
OH2
(а)
(b)
(c)
(d)
6. When HBr is added to 1-butene (as shown in the following reaction equation) the
newly formed stereocenter must be a racemic mixture of both R & S forms
because (a) 1-butene forms a 1° carbocation, (b) the intermediate 2° carbocation
can be attacked from either face, (c) the intermediate 2° carbocation rearranges
by 1,2 -hydride shifts before the Br- attacks, (d) the two products are achiral
:Brt
CH CH
CH,
(S)-2-bromobutane
HBr
CH,
Planar carbocation
CH CH
CH,CH
:Bet
CH
:Be:
(R)-2-bromobutane
7. In the reaction shown below which converts an alkene to an alcohol, what species
acts as the nucleophile in the reaction with the carbocation intermediate?
(a) a sulfuric acid molecule, (b) a hydronium ion (c) a neutral water molecule,
(d) the C=C of the alkene.
slow
R-CH=CH2 + H-OSO3H
R-CHCH3
H20
R-CHCH3
R-CHCH3 + H3O:
SHCHS
HO:
H-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning