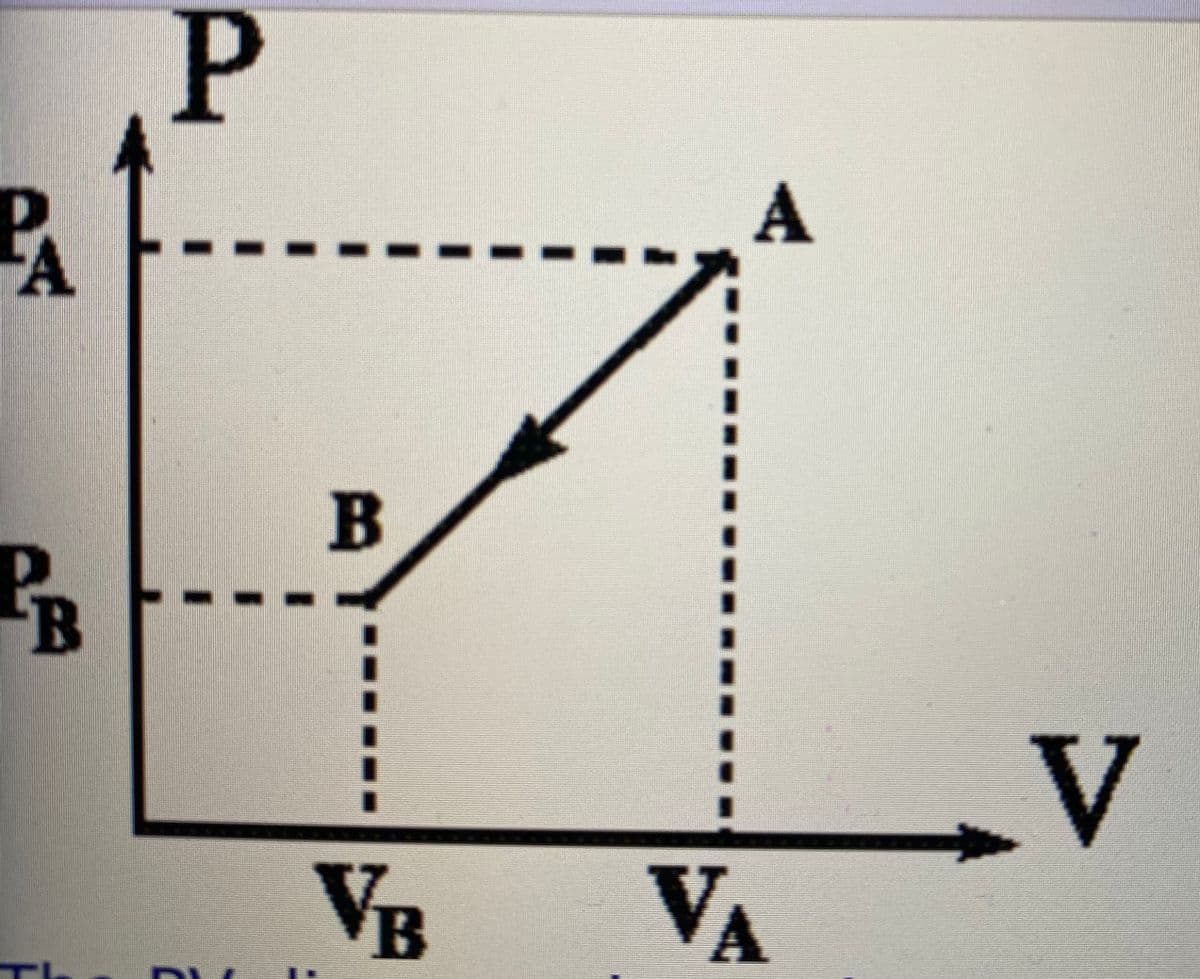
The PV diagram shows the compression of 40.9 moles of an ideal monoatomic gas from state A to state B. Calculate Q, the heat added to the gas in the process A to B. Data: PA= 1.90E+5 N/m2 VA= 1.83E+0 m3 PB= 1.01E+5 N/m2 VB= 8.90E-1 m3
The PV diagram shows the compression of 40.9 moles of an ideal monoatomic gas from state A to state B. Calculate Q, the heat added to the gas in the process A to B. Data: PA= 1.90E+5 N/m2 VA= 1.83E+0 m3 PB= 1.01E+5 N/m2 VB= 8.90E-1 m3
Chapter11: Combined Structure Problems
Section: Chapter Questions
Problem 31P
Related questions
Question
The PV diagram shows the compression of 40.9 moles of an ideal monoatomic gas from state A to state B. Calculate Q, the heat added to the gas in the process A to B. Data: PA= 1.90E+5 N/m2 VA= 1.83E+0 m3 PB= 1.01E+5 N/m2 VB= 8.90E-1 m3›44
Transcribed Image Text:P
%3D
%3D
.V
VB
VA
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

