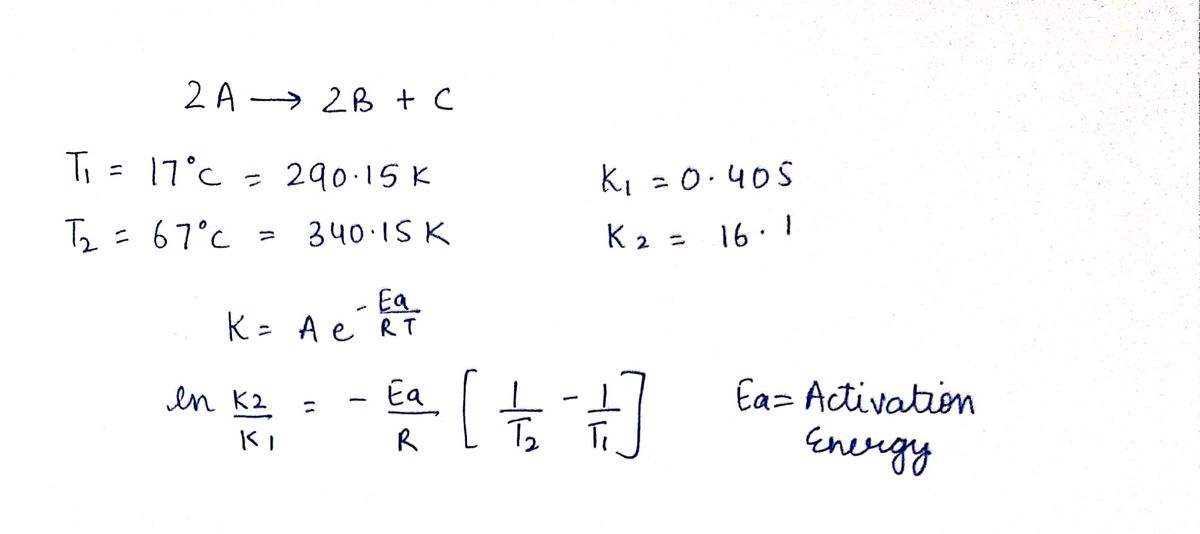
The rate constants for the first order reaction 2 A - 2 8 + Cwere found at different temperatures (below). What is the activation energy for this reaction in k/mol? T1 = 17 °c k1 = 0.405 T2 = 67 °C k2 = 16.1 Report your answer the nearest whole number k2 In- R T2 k = Ae %3B
The rate constants for the first order reaction 2 A - 2 8 + Cwere found at different temperatures (below). What is the activation energy for this reaction in k/mol? T1 = 17 °c k1 = 0.405 T2 = 67 °C k2 = 16.1 Report your answer the nearest whole number k2 In- R T2 k = Ae %3B
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter12: Kinetics
Section: Chapter Questions
Problem 82E: For each of the following reaction diagrams, estimate the activation energy (Ea) of the reaction:
Related questions
Question

Transcribed Image Text:The rate constants for the first order reaction 2 A - 2 8 + Cwere found at different temperatures (below). What is the activation energy for this reaction in k/mol?
T1 = 17 °c
k1 = 0.405
T2 = 67 °C
k2 = 16.1
Report your answer the nearest whole number
k2
In-
R T2
k = Ae
%3B
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning