The rate of reaction in terms of the "rate law expression" includes the rate constant (k), the concentration of the reactants, and the orders of the reaction with respect to the different reactants. Consider the following reaction: A +B→C+D The initial concentrations of the reactants A and B are 0.400 M and 0.350 M, respectively. The rate of reaction is 0.060 M ·s, and the orders of the reaction, with respect to reactants A and B, are 1 and 2, respectively. Determine the rate constant (k) for the reaction using the rate law. -1 Express your answer in M²·s to three significant figures. • View Available Hint(s) μνα ΑΣφ -2 The rate constant(k) = %3D
The rate of reaction in terms of the "rate law expression" includes the rate constant (k), the concentration of the reactants, and the orders of the reaction with respect to the different reactants. Consider the following reaction: A +B→C+D The initial concentrations of the reactants A and B are 0.400 M and 0.350 M, respectively. The rate of reaction is 0.060 M ·s, and the orders of the reaction, with respect to reactants A and B, are 1 and 2, respectively. Determine the rate constant (k) for the reaction using the rate law. -1 Express your answer in M²·s to three significant figures. • View Available Hint(s) μνα ΑΣφ -2 The rate constant(k) = %3D
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter14: Chemical Kinetics: The Rates Of Chemical Reactions
Section: Chapter Questions
Problem 12PS: The reaction 2 NO(g) + 2 H2(g) N2(g) + 2 H2O(g) was studied at 904 C, and the data in the table...
Related questions
Question
100%
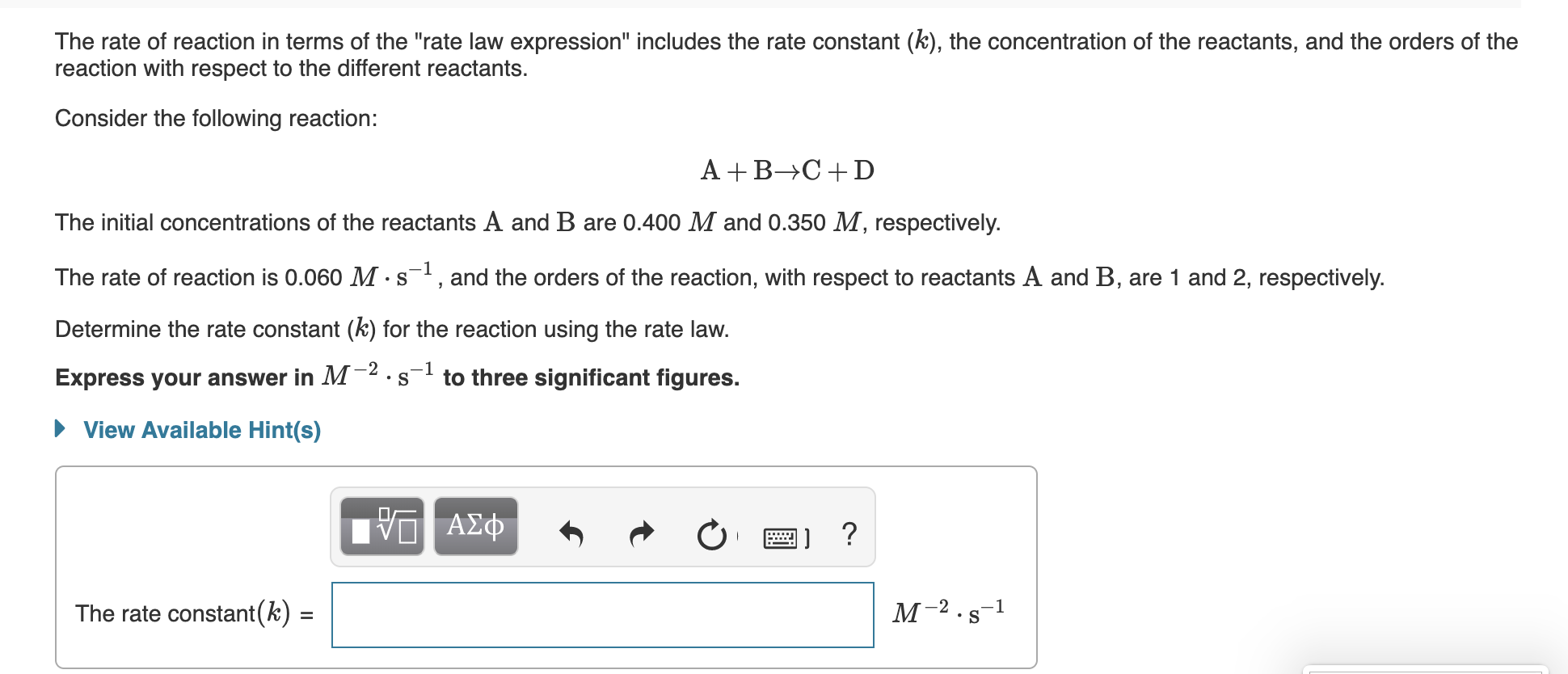
Transcribed Image Text:The rate of reaction in terms of the "rate law expression" includes the rate constant (k), the concentration of the reactants, and the orders of the
reaction with respect to the different reactants.
Consider the following reaction:
A +B→C+D
The initial concentrations of the reactants A and B are 0.400 M and 0.350 M, respectively.
The rate of reaction is 0.060 M ·s, and the orders of the reaction, with respect to reactants A and B, are 1 and 2, respectively.
Determine the rate constant (k) for the reaction using the rate law.
-1
Express your answer in M²·s
to three significant figures.
• View Available Hint(s)
μνα ΑΣφ
-2
The rate constant(k) =
%3D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 5 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning