The reaction of methane with water to form carbon dioxide and hydrogen is non-spontaneous at 298 K. At what temperature will this system make the transition from non-spontaneous to spontaneous? The data refer to 298 K. CH:(g) + 2H2O(g) * CO2(g) + 4H2(g) Substance: CH4(g) H2O(g) CO:(g) H2(g) AH® {(kJ/mol): AG° f (kJ/mol): S°(J/K mol): -74.87 -241.8 -393.5 f ww -50.81 -228.6 -394.4 ww 186.1 188.8 213.7 130.7
The reaction of methane with water to form carbon dioxide and hydrogen is non-spontaneous at 298 K. At what temperature will this system make the transition from non-spontaneous to spontaneous? The data refer to 298 K. CH:(g) + 2H2O(g) * CO2(g) + 4H2(g) Substance: CH4(g) H2O(g) CO:(g) H2(g) AH® {(kJ/mol): AG° f (kJ/mol): S°(J/K mol): -74.87 -241.8 -393.5 f ww -50.81 -228.6 -394.4 ww 186.1 188.8 213.7 130.7
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter18: Thermodynamics And Equilibrium
Section: Chapter Questions
Problem 18.120QP
Related questions
Question
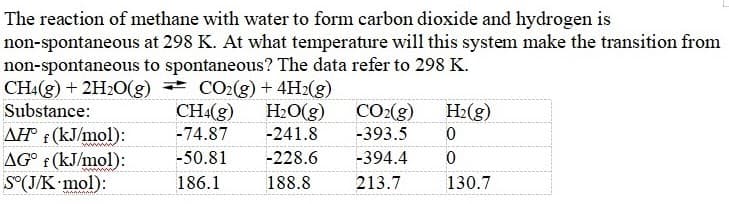
Transcribed Image Text:The reaction of methane with water to form carbon dioxide and hydrogen is
non-spontaneous at 298 K. At what temperature will this system make the transition from
non-spontaneous to spontaneous? The data refer to 298 K.
CH4(g) + 2H2O(g) CO2(g)+ 4H2(g)
Substance:
CH(g)
H2O(g)
CO:(g)
H2(g)
AH® ¢ (kJ/mol):
AG° f (kJ/mol):
S°(J/K mol):
-74.87
-241.8
-393.5
ww
-50.81
-228.6
-394.4
www
186.1
188.8
213.7
130.7
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
