The Robinson carbonyl coupling reactions, the Michael reaction and the intramolecular aldol condensation. The most common partners in the li donor (such as a cyclic ketone, a β-keto ester, an enamine, or a β-diketone) and an α,β-unsaturated ketone acceptor. The product is a substituted 2-cyclohexenone derivative annulation is a combination of two reaction are a nucleophi Draw the structures of the nucleophilic B-keto ester and the α,β-unsaturated ketone acceptor that were combined in a Robinson annulation to give the following product Ph Ph Ph Ph CO2Et Draw the B-keto ester as an ethyl ester
The Robinson carbonyl coupling reactions, the Michael reaction and the intramolecular aldol condensation. The most common partners in the li donor (such as a cyclic ketone, a β-keto ester, an enamine, or a β-diketone) and an α,β-unsaturated ketone acceptor. The product is a substituted 2-cyclohexenone derivative annulation is a combination of two reaction are a nucleophi Draw the structures of the nucleophilic B-keto ester and the α,β-unsaturated ketone acceptor that were combined in a Robinson annulation to give the following product Ph Ph Ph Ph CO2Et Draw the B-keto ester as an ethyl ester
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter19: Enolate Anions And Enamines
Section19.9: Crossed Enolate Reactions Using Lda
Problem BQ
Related questions
Question
The Robinson annulation is a combination of two carbonyl coupling reactions, the Michael reaction and the intramolecular aldol condensation. The most common partners in the reaction are a nucleophilic donor (such as a cyclic
Draw the structures of the nucleophilic β-keto ester and the α,β-unsaturated ketone acceptor that were combined in a Robinson annulation to give the following product:
- Draw the β-keto ester as an ethyl ester,
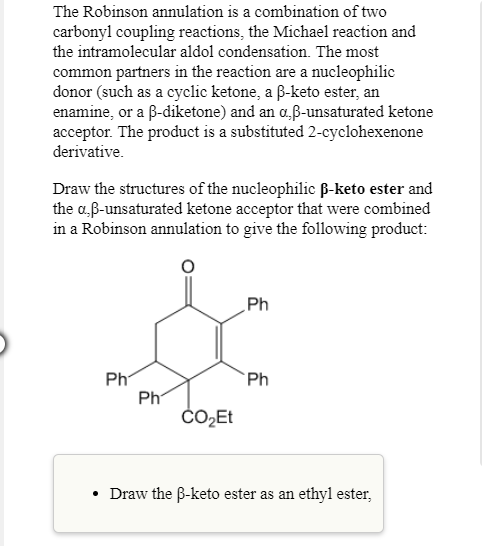
Transcribed Image Text:The Robinson
carbonyl coupling reactions, the Michael reaction and
the intramolecular aldol condensation. The most
common partners in the li
donor (such as a cyclic ketone, a β-keto ester, an
enamine, or a β-diketone) and an α,β-unsaturated ketone
acceptor. The product is a substituted 2-cyclohexenone
derivative
annulation is a combination of two
reaction are a nucleophi
Draw the structures of the nucleophilic B-keto ester and
the α,β-unsaturated ketone acceptor that were combined
in a Robinson annulation to give the following product
Ph
Ph
Ph
Ph
CO2Et
Draw the B-keto ester as an ethyl ester
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning