Q: The density of a substance is defined as the ratio of the mass to the volume for that substance. Use…
A: A question based on properties of solid that is to be accomplished.
Q: (d) Br tok
A: The above reaction will most likely to proceed through E2 mechanism where E2 stand for bimolecular…
Q: In the blank given, indicate if the absolute stereochemistry of each chiral carbon is R or S. Br S…
A:
Q: Salicylic Acid Test. Methyl alcohol with salicylic acid and concentrated sulfuric acid will give…
A:
Q: Choose whether Atomic Absorption Spectroscopy (AAS) or Gas Chromatography Mass Spectrometry (GCMS)…
A: Here we have to determine which method should we use between AAS and GC-MS for the detection of the…
Q: Give the IUPAC names
A: Requirement from question: Need to write IUPAC name of given three componds.
Q: to write ionic equation for the reaction of acetic acid with itheim bromide, and specify Rwhether…
A: Starting materials Acetic acid : CH3COOH Lithium bromide : LiBr pKa of acetic acid = 4.7 pKa of…
Q: .OH 1. NaH 2. CH3CH2CH2Br
A: Alcohol react with base (NaH) deprotonation occurred (alkoxide formed) Alkoxide undergo substitution…
Q: Draw the major product of the substitution reaction shown below. Ignore any inorganic byproducts.…
A: We have to draw the SN1 product.
Q: TUTOR Concentration of Unknown via Titration I 44.58 mL of a solution of the acid H₂C₂O4 is…
A: Given : Volume of NaOH solution= 42.80 ml Concentration of NaOH solution= 0.6900 M Volume of…
Q: H3C НО OCH3 ОН тос H3C НО LOCH 3 OH
A: Requirement from question: Four questions. Identify whether they are stereoisomers, constitutional,…
Q: Given the following reactions N2 (g) +202 (g) → 2NO2 (g) 2NO(g) + O2 (g) → 2NO2 (g) ΔΗ = -114.2 kJ…
A:
Q: [Review Topics] [References] Specify the local electron geometries about the atoms labelled a-d.…
A: Determine geometry at a,b,c and d ?
Q: Give the name for the product from the hydrogenation of each of the following. 3-methyl-2-hexene…
A:
Q: A piece of metal weighs 8.25 g. When a student places it into a graduated cylinder containing water,…
A: we have to calculate the density of metal
Q: If a penny is made of 3.11 grams of copper, how many atoms of copper are there in the penny?…
A:
Q: How many stereoisomers (including itself) does the following molecule have? Br que OH 04 016 02 03…
A:
Q: QUESTION Complete the following synthetic transformation by selecung from the list of reagents below…
A:
Q: Hydroboration of alkenes is an example of: O a. a substitution reaction O b. a free radical action…
A:
Q: Predict the two most likely mechanisms for the reaction of 2-lodohexane with sodium ethoxide. OA SN2…
A:
Q: write an acyclic organic molecule that contains exactly two tertiary hydrogen atoms.
A: The stability of primary, secondary and tertiary carbon atoms in an organic molecule is determined…
Q: H₂O2(aq) + 21 (aq) + 2H+ (aq) → 12(aq) + 2H₂00 -
A: Here is explanation for rate determining step and stoichiometry equation.
Q: Draw the structural condensed formula of methyl pentanoate.
A:
Q: Phosphorus pentachloride decomposes according to the chemical equation…
A:
Q: 16. Circle and write the name of all the functional groups in the following structure. 0 H -OH 0…
A: A functional group is a group of atoms in a molecule with distinct chemical properties.
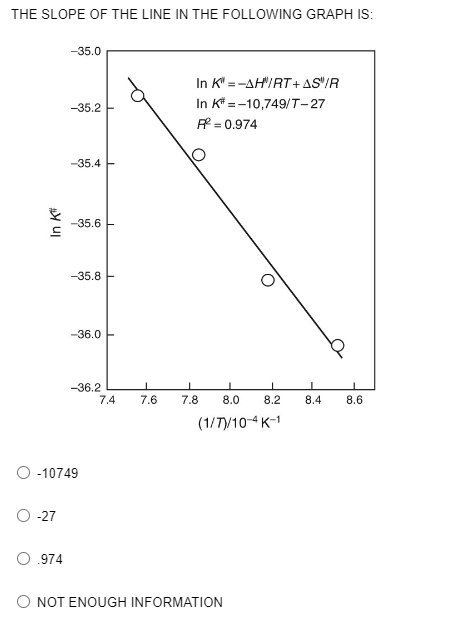
Q: What is the enthalpy of vaporization of this liquid? Δ?vap=
A: According to Clausius-Clapeyron equation, ln P =-∆HvapR(1T) + C For two temperatures, it can be…
Q: The indicator used in the Titration of Acids and Bases lab was methyl orange. True False
A: INDICATOR:-Indicator is a substance which changes its colour in acidic and basic medium. -So these…
Q: Draw the major organic product of the reaction shown. OH HI Incorrect H
A:
Q: Predict the major organic product of the following reactions or provide the reagents needed o…
A: Given : structure of reactant and products.
Q: Arrange the following carbocations in order of their decreasing stability (most stable first). va A…
A: Carbocation is a electron deficient species.
Q: Provide the correct systematic name for the compound shown here. H3C NH₂ CH3 CH3
A: Write IUPAC name of the given structures--
Q: Write the balanced COMPLETE ionic equation for the reaction when aqueous aluminum nitrate and…
A: Ionic equation involves all the ions of the reactants along with the product and ions on the product…
Q: 1. The study of the amounts or reactants used, and products formed from a chemical reaction is…
A: Since you have asked multiple questions, we will solve the first question for you. If you want any…
Q: When allyl bromide is refluxed with magnesium metal in ether solvent, the product formed is…
A: At first grignard reagent is formed, and it become nucleophilic, so further attack in allyl bromide…
Q: Why do we not see uranium and thorium in the core even though they are heavier elements
A: If uranium or thorium will be present at earth core then under high temperature and pressure,…
Q: Add one or more curved arrows to show the movement of electrons in the reaction. To draw the arrows,…
A: Answer of this question :- It is Homo nuclear atom so in this molecule, Homolytic bond fission take…
Q: 0000 Select the correct Newman projection looking down the C2-C3 bond of the below structure. CI ОА…
A:
Q: Complete the nuclear reactions. Then write each equation in either expanded or short form. a)…
A: Answer:- a. 63Li + 21H -> 74Be + 10n Sum of atomic mass both side reaction = 8…
Q: Give the set of four quantum numbers that could represent the last electron added (using the the…
A:
Q: Question 28 The half-life of 14C is 5,730 yr Assuming some charcoal from a campfire fraction of the…
A: We have to predict the age of piece of carbon.
Q: What are the potential errors that could occur when using the Kjeldahl method to determine the…
A: Kjeldahl method principle : Strong acid helps in the digestion of food and liberates nitrogen which…
Q: The four hydrogen bonds within each ß-sheet in the amyloid-like fibril occur between the following…
A: Beta sheet is a secondary structure of protein formed when several beta strands self assemble in…
Q: Draw the substitution product formed in the reaction. Draw the product. CH3 CH3 CH3 H3C-C * + H₂C C…
A: we have to draw the substitution product for the given reaction
Q: 1-The boiling point of dimethyl sulfoxide at 1 atm is 190 oC. Use the boiling point nomograph to…
A:
Q: H ball & stick V Strain energy = H H labels Previous
A: There is no strain between gauch CH3:H Eclipse H:H = 1kcal/mol
Q: How many moles of AgCl are contained in 244 mL of 0.135 m AgCl solution? The density of the solution…
A:
Q: the ΔG'° of the reaction A → B is –40 kJ/mol, under standard conditions the reaction: a. is at…
A: Given -> A <------> B. ∆G°= -40 KJ/mole
Q: For the following reaction, 20.9 grams of iron are allowed to react with 9.42 grams of oxygen gas.…
A:
Q: Ibuprofen is a chiral compound used as a nonsteroidal anti-inflammatory. Determine the number of…
A: Since you have asked multiple questions, we will solve the first question for you. If you want any…
Q: Consider these structures to answer the following three questions 0 A. CH; -C-CIT Br B. H Xe Br C.…
A: SN1 (1) Polar protic solvent (2) Weak nucleophile (3) Stability of carbocation (3) Stability: 3o…
Step by step
Solved in 2 steps with 2 images

- The atomic hydrogen exists in space at an estimated concentration of one particle per cubic meter. If the collision diameter is 2.5 ×10^(–10) meter and the temperature is 2.7 Kelvin, how many kilometers away will the next potential collision be?For the reduction 2FeCl3 + SnCl2 =====➔ 2 FeCl2 + SnCl4 in aqueous solution the following data were obtained at 25oC t(min) 1 3 7 11 40 Y 0.01434 0.02664 0.03612 0.04102 0.05058 Where y is the amount of FeCl3 reacted in moles per liter. The initial concentrations of SnCl3 and FeCl3 were respectively, 0.03125, 0.0625 moles/L. a.)Show that the reaction is third order (derive the rate law), and b.) calculate the average specific rate constant.The percentage protein content of chicken breasts are determined 5 times and the following results obtained: 28.5, 30.1, 30.2, 30.0 en 30.2%. The first value (28.5%) appears anomalous. Should it be retained or rejected at a 90% confidence level? Show all equations.
- Chemistry An aquifer contaminated with petroleum is found to have the following component concentrations at a particular site: benzene158 ppm toluene124 ppm ethylbenzene91 ppm xylene45 ppm n-heptadecane161 ppm pristane 84 ppm Provide an estimate for the age of the spill at this site using (a) BTEX ratio and (b) nC17:Pr ratio. Show your calculations and use units throughout. Give proper s.f. for the answer.The atomic hydrogen exists in space at an estimated concentration of one particle per cubic meter. If the collision diameter is 2.5 ×10^(–10) meter and the temperature is 2.7 Kelvin, how many kilometers away will the next potential collision be? Express the answer in three significant figures.I AM NOT A NATIVE ENGLISH SPEAKER, PLS WRITE IT CLEAR. The following is a formulation of the decomposition of penta-nitrogen dioxide in 456K N2O5(g) → 2NO2(g) +1\2O2(g) ? = 6.35 × 10−3?−1 A 0.18 liter hard vessel located in the 456K was inserted N2O5(G) At a pressure of 1.22 atmospheres. the reaction above happend. 1. How long from the start of the experiment will the pressure in the system rise to 1.54 atmospheres? 2. Calculate the partial pressure of each component in the mixture 100 seconds from the start of the experiment. 3. It was found that at a temperature of K 400 the value of the rate constant for a decomposition reaction of a nitrogen Fanta-oxygen equals 5.42*10-3 sec-1. Calculate the operating energy for the reaction. in all sections show calculations.
- A chemist obtained the following data for percent lindane in the triplicate analysis of an insecticide preparation: 7.23, 6.95, and 7.53%. Calculate the 90% confidence interval for the mean of the the three data, assuming that (a) the only information about the precision of the method is the precision for the three data. (b) on the basis of long experience with the method, it is believed that s---->σ lindane. (c) If s=0.28 is good estimate of σ, how many replicate measurement should be made in order for the mean for the analysis of sample to be within 0.2% of the true mean 90% of the time.Potassium dichromate has several industrial applications. To determine the purity of the salt that will be used in different industrial processes, a sample mass equal to 2.660 g was dissolved and quantitatively transferred to a 500.00 mL flask. An aliquot of 25.00 mL of this solution was treated with excess KI and the released iodine was titrated with 0.1000 mol L-1 sodium thiosulfate, spending 27.00 mL. Calculate the purity of the analyzed salt. Data:K = 39.10 O = 16.00 Cr = 52.00 I = 126.9 S = 32.07The slope of an Arrhenius plot was found to be -4570 K, and the intercept was found to be 3.3 with the time plotted in seconds. What is the frequency factor? Enter your answer with two sig figs.
- Three large proteins are ionized at the pH at which an electrical FFF separation is carried out. (i) if the ions are designated A2+, B2+, and C3+, predict the order of elution. (ii) What is FFF? (iii) List the four subtechniques of FFF.2AB2(g) = A2(g) + 2B2(g) A 500,0 ml ask is filled with 0,384 mol of AB2. the appearance of A2 is monitored at timed intervals. assume that temprature and volume are kept constant. the data obtained are shown in the table below. time/min : 0 10 20 30 40 50 moles of A2 : 0 0,0541 0,0833 0,1221 0,1432 0,1567 a. make a similiar table for the dissapearance of AB2 b. what is the average rate of dissapearance of AB2 over the second and the third 10 minutes intervals? c. what is the average rate of appearance of A2 between t=30 and t=50?kindly help me with this problem Thank you! Subject: Physical Chemistry Instructions: solve as neatly as possible and show complete solution.No rounding-off for your decimals (include all decimals in your calculator in your computation). The following data were obtained for the adsorption of acetone on charcoal from an aqueous solution at 180C. Y (millimoles/g) C (millimoles/liter) 0.208 2.34 0.618 14.65 1.075 41.03 1.5 88.62 2.08 177.69 2.88 268.97 a) Find the constants k and n of the Freundlich equation. b) Find the constants a and b in the Langmuir equation. c) Find the constants k and n in the Temkin isotherm. d) Which of the isotherm fit the data best?



