The specific heat capacities for several substances are shown in the table. Substance Specific Heat (J/g•°C) iron 0.45 air 1.01 water 4.18 glass 0.84 If there are equal masses of each substance, which substance must absorb the greatest amount of heat to increase the temperature by the same amount?
The specific heat capacities for several substances are shown in the table. Substance Specific Heat (J/g•°C) iron 0.45 air 1.01 water 4.18 glass 0.84 If there are equal masses of each substance, which substance must absorb the greatest amount of heat to increase the temperature by the same amount?
Living By Chemistry: First Edition Textbook
1st Edition
ISBN:9781559539418
Author:Angelica Stacy
Publisher:Angelica Stacy
ChapterU5: Fire: Energy , Thermodynamics, And Oxidation-reduction
SectionU5.5: The Heat Is On: Specific Heat Capacity
Problem 1E
Related questions
Question
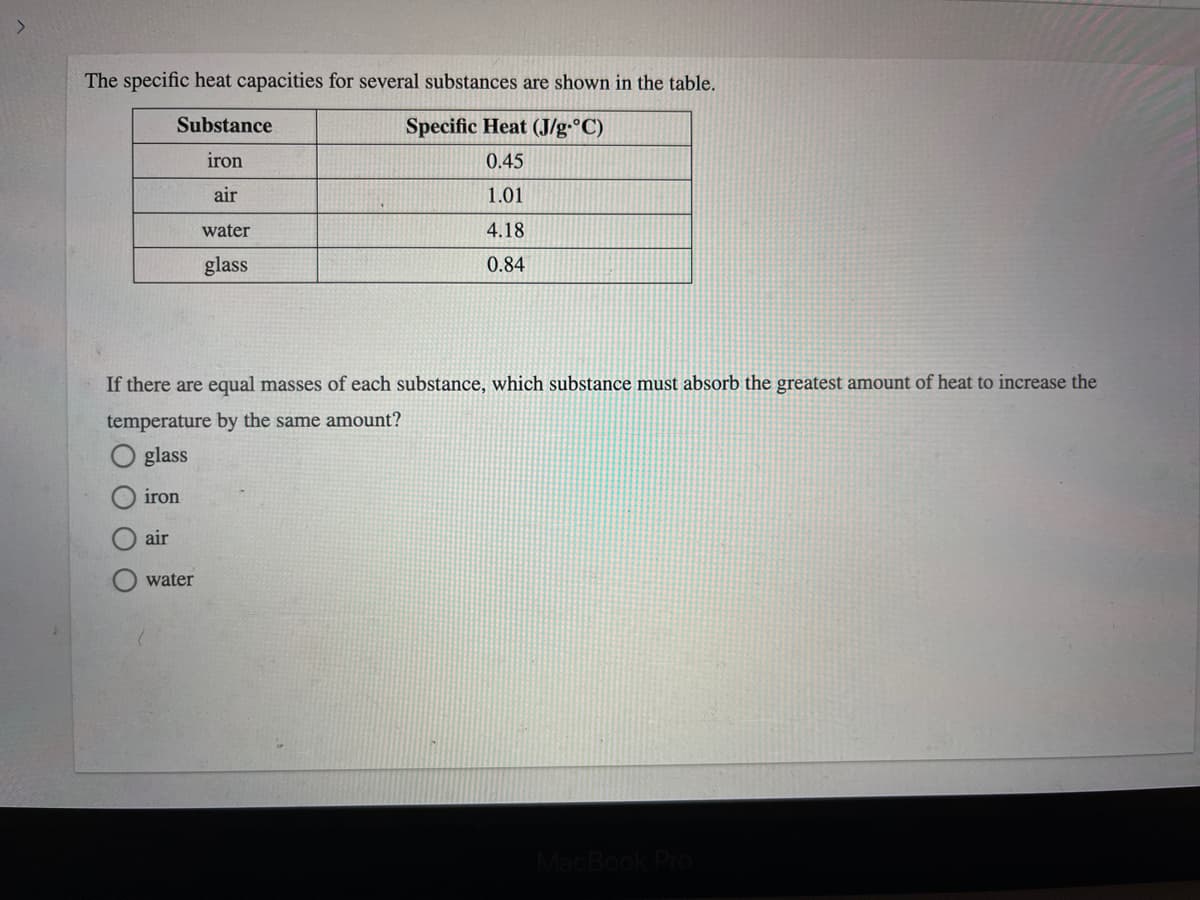
Transcribed Image Text:The specific heat capacities for several substances are shown in the table.
Substance
Specific Heat (J/g•°C)
iron
0.45
air
1.01
water
4.18
glass
0.84
If there are equal masses of each substance, which substance must absorb the greatest amount of heat to increase the
temperature by the same amount?
glass
iron
air
water
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning