The student collected the following data he prepared solutions ranging from 0.000 to 0.500 ppm and measured the instrumental reading corresponding to each of the solutions prepared: Xi yi 0.000 0.00 0.100 12.36 0.200 24.83 0.300 35.91 0.400 48.79 0.500 60.42 i) Use the method of least squares to calculate the equation of the best straight line going through these
The student collected the following data he prepared solutions ranging from 0.000 to 0.500 ppm and measured the instrumental reading corresponding to each of the solutions prepared: Xi yi 0.000 0.00 0.100 12.36 0.200 24.83 0.300 35.91 0.400 48.79 0.500 60.42 i) Use the method of least squares to calculate the equation of the best straight line going through these
Chapter3: Statistical Tests With Excel
Section: Chapter Questions
Problem 1P
Related questions
Question
solve i
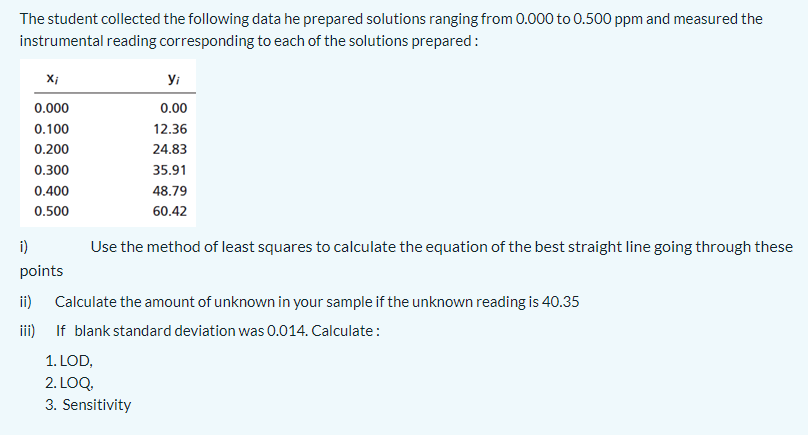
Transcribed Image Text:The student collected the following data he prepared solutions ranging from 0.000 to 0.500 ppm and measured the
instrumental reading corresponding to each of the solutions prepared:
yi
0.000
0.00
0.100
12.36
0.200
24.83
0.300
35.91
0.400
48.79
0.500
60.42
i)
Use the method of least squares to calculate the equation of the best straight line going through these
points
ii) Calculate the amount of unknown in your sample if the unknown reading is 40.35
iii) If blank standard deviation was 0.014. Calculate:
1. LOD,
2. LOQ,
3. Sensitivity
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you







Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning