The successive ionization energies of a certain element are The 1st ionization energy- 801 kJ/mol The 2nd ionization energy = 2427 kJ/mol The 3rd ionization energy 3659 kJ/mol The 4th ionization energy 25022 kJ/mol The 5th ionization energy 32822 kJ/mol. Determine the identity of the unknown element that suggests this pattern of ionization energies. OF O Si OB O Na Be Att 2
The successive ionization energies of a certain element are The 1st ionization energy- 801 kJ/mol The 2nd ionization energy = 2427 kJ/mol The 3rd ionization energy 3659 kJ/mol The 4th ionization energy 25022 kJ/mol The 5th ionization energy 32822 kJ/mol. Determine the identity of the unknown element that suggests this pattern of ionization energies. OF O Si OB O Na Be Att 2
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter6: The Structure Of Atoms
Section: Chapter Questions
Problem 38PS: Explain briefly why each of the following is not a possible set of quantum numbers for an electron...
Related questions
Question
100%
Please give me all answers
I am upvote for you other wise down vote given any miss.
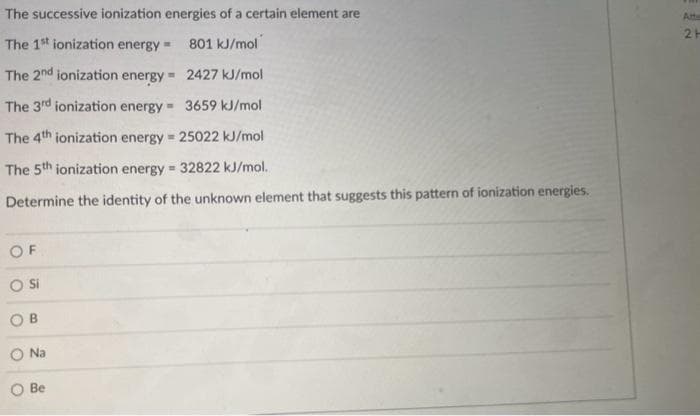
Transcribed Image Text:The successive ionization energies of a certain element are
The 1st ionization energy
801 kJ/mol
The 2nd ionization energy =
2427 kJ/mol
The 3rd ionization energy = 3659 kJ/mol
The 4th ionization energy = 25022 kJ/mol
The 5th ionization energy = 32822 kJ/mol.
Determine the identity of the unknown element that suggests this pattern of ionization energies.
OF
O Si
OB
Na
Be
Atte
2H

Transcribed Image Text:For each set of quantum numbers, determine if it is allowed to describe an electron.
1. n=2
/ =
/= 1
1
m₁ = +1
II.
n = 3
1 = 2
m₁ = -1
III. n = 5
1 = 4
m₁ = +1
O (I) and (III) are allowed.
O Only (III) is allowed.
O Only (1) is allowed.
O Only (II) is allowed.
O (I) and (II) are allowed.
A gaseous sample has energy (E) of 1.02 kJ. The volume and the pressure of the gas are 1.00 L and
1.00 atm, respectively.
Determine the enthalpy of the system.
O-102.3 kJ
O 102.3 kJ
O 1.12 kJ
m₂ = +1/2
m, = 0
m, = -1/2
0-97.6 kJ
O 0.92 kJ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning