The surface area of 1.0 g alumina was determined by adsorption of nitrogen at -196°C. At pressure of 37 torr and 115 torr, the volume of nitrogen adsorbed was 23 cm3 and 33 cm3, respectively. By using the following BET equation: Calculate the surface area of the alumina when the given saturation pressure, Po is 760 torr and molecular area of nitrogen is 16.2 x 10-20 m2 b) If argon gas is used as an adsorbate, would the surface area of the alumina be the same as calculated above? Explain your answer.
The surface area of 1.0 g alumina was determined by adsorption of nitrogen at -196°C. At pressure of 37 torr and 115 torr, the volume of nitrogen adsorbed was 23 cm3 and 33 cm3, respectively. By using the following BET equation: Calculate the surface area of the alumina when the given saturation pressure, Po is 760 torr and molecular area of nitrogen is 16.2 x 10-20 m2 b) If argon gas is used as an adsorbate, would the surface area of the alumina be the same as calculated above? Explain your answer.
Chapter14: Chromatography
Section: Chapter Questions
Problem 9P
Related questions
Question
a) The surface area of 1.0 g alumina was determined by adsorption of nitrogen at -196°C. At pressure of 37 torr and 115 torr, the volume of nitrogen adsorbed was 23 cm3 and 33 cm3, respectively. By using the following BET equation:
Calculate the surface area of the alumina when the given saturation pressure, Po is 760 torr and molecular area of nitrogen is 16.2 x 10-20 m2
b) If argon gas is used as an adsorbate, would the surface area of the alumina be the same as calculated above? Explain your answer.
answer b only
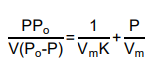
Transcribed Image Text:PP.
1
V(Po-P) VmK Vm
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



