The surface of an ideal molecule of table salt (NaCl) is a square with a chemical structure as shown in the figure. The blue spheres represent Na+ ions and the green spheres represent Clions. Consider only points A, B, C, and D. What is the magnitude of the net electrostatic force on the chlorine ion at point A due to the sodium ions at points B and C? Note: the bond length is 0.282 nm. N D C A I
The surface of an ideal molecule of table salt (NaCl) is a square with a chemical structure as shown in the figure. The blue spheres represent Na+ ions and the green spheres represent Clions. Consider only points A, B, C, and D. What is the magnitude of the net electrostatic force on the chlorine ion at point A due to the sodium ions at points B and C? Note: the bond length is 0.282 nm. N D C A I
Chapter6: Nuclear Magnetic Resonance Spectroscopy Part Two: Carbon-13 Spectra, Including Heteronuclear Coupling With Other Nuclei
Section: Chapter Questions
Problem 22P
Related questions
Question
100%
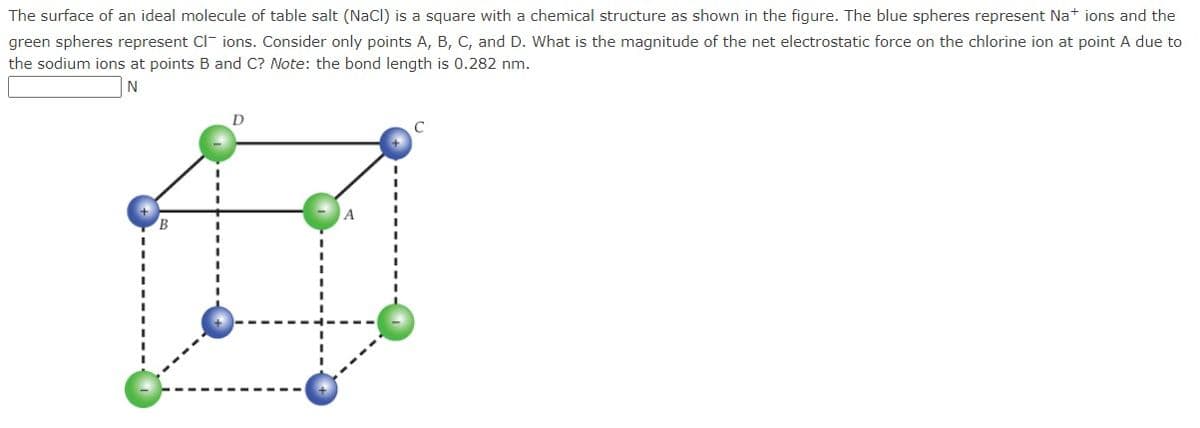
Transcribed Image Text:The surface of an ideal molecule of table salt (NaCl) is a square with a chemical structure as shown in the figure. The blue spheres represent Na+ ions and the
green spheres represent Clions. Consider only points A, B, C, and D. What is the magnitude of the net electrostatic force on the chlorine ion at point A due to
the sodium ions at points B and C? Note: the bond length is 0.282 nm.
N
D
A
C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


Modern Physics
Physics
ISBN:
9781111794378
Author:
Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


Modern Physics
Physics
ISBN:
9781111794378
Author:
Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning