The temperature is raised. Heat can be considered a reactant in an exothe reaction will shift to the right. As the temperatur ic reaction and a product in an endothermic reaction. As the temperature is increased, an endothermic increased, an exothermic reaction will shift to the left. Heat can be considered a reactant in an endoth reaction will shift to the right. As the temperature s increased, an exothermic reaction will shift to the left. mic reaction and a product in an exothermic reaction. As the temperature is decreased, an endothermic Heat can be considered a reactant in an endothermic reaction and a product in an exothermic reaction. As the temperature is increased, an endothermic reaction will shift to the right. As the temperature is increased, an exothermic reaction will shift to the left. Heat can be considered a reactant in an endothermic reaction and a product in an exothermic reaction. As the temperature is increased, an endothermic reaction will shift to the left. As the temperature is decreased, an exothermic reaction will shift to the left.
The temperature is raised. Heat can be considered a reactant in an exothe reaction will shift to the right. As the temperatur ic reaction and a product in an endothermic reaction. As the temperature is increased, an endothermic increased, an exothermic reaction will shift to the left. Heat can be considered a reactant in an endoth reaction will shift to the right. As the temperature s increased, an exothermic reaction will shift to the left. mic reaction and a product in an exothermic reaction. As the temperature is decreased, an endothermic Heat can be considered a reactant in an endothermic reaction and a product in an exothermic reaction. As the temperature is increased, an endothermic reaction will shift to the right. As the temperature is increased, an exothermic reaction will shift to the left. Heat can be considered a reactant in an endothermic reaction and a product in an exothermic reaction. As the temperature is increased, an endothermic reaction will shift to the left. As the temperature is decreased, an exothermic reaction will shift to the left.
Chapter13: Chemical Equilibrium
Section: Chapter Questions
Problem 10RQ: The only stress (change) that also changes the value of K is a change in temperature. For an...
Related questions
Question
Transcribed Image Text:Part D
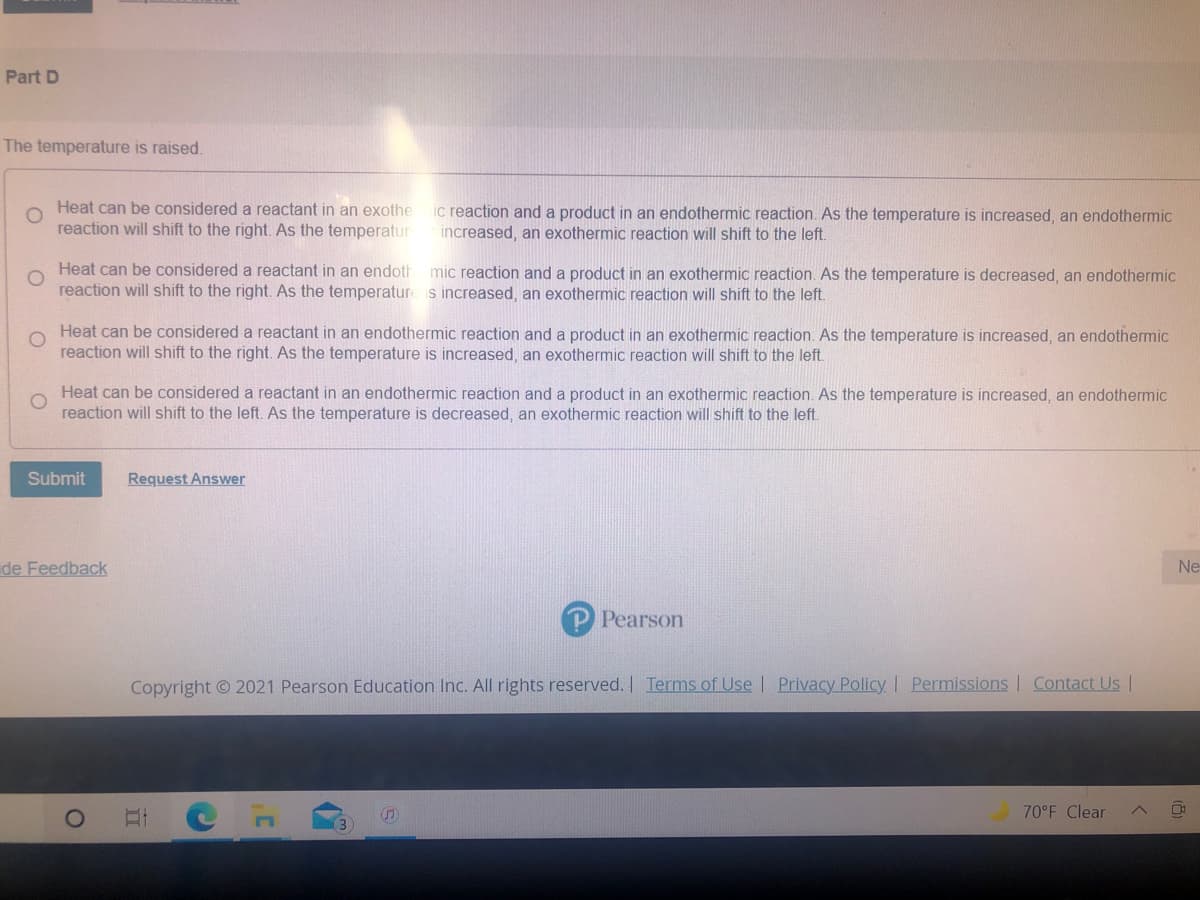
The temperature is raised.
Heat can be considered a reactant in an exothe
reaction will shift to the right. As the temperatur
ic reaction and a product in an endothermic reaction. As the temperature is increased, an endothermic
increased, an exothermic reaction will shift to the left.
Heat can be considered a reactant in an endoth
reaction will shift to the right. As the temperature s increased, an exothermic reaction will shift to the left.
mic reaction and a product in an exothermic reaction. As the temperature is decreased, an endothermic
Heat can be considered a reactant in an endothermic reaction and a product in an exothermic reaction. As the temperature is increased, an endothermic
reaction will shift to the right. As the temperature is increased, an exothermic reaction will shift to the left.
Heat can be considered a reactant in an endothermic reaction and a product in an exothermic reaction. As the temperature is increased, an endothermic
reaction will shift to the left. As the temperature is decreased, an exothermic reaction will shift to the left.
Submit
Request Answer
de Feedback
Ne-
Pearson
Copyright 2021 Pearson Education Inc. All rights reserved. Terms of Use | Privacy Policy Permissions | Contact Us |
(5)
70°F Clear
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning