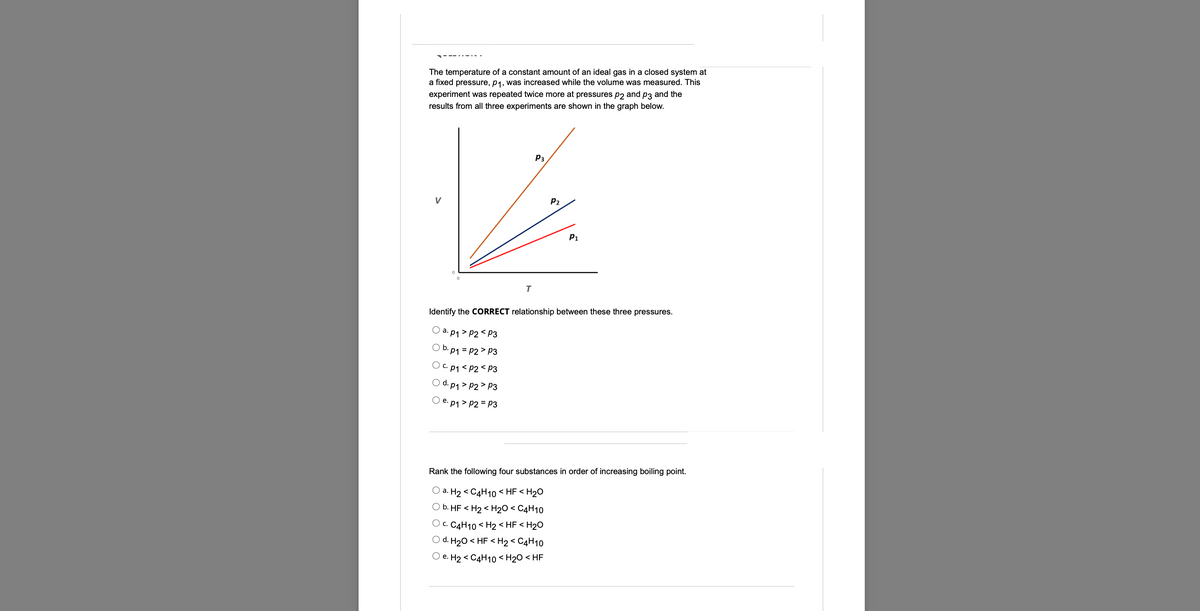
The temperature of a constant amount of an ideal gas in a closed system at a fixed pressure, p₁, was increased while the volume was measured. This experiment was repeated twice more at pressures p2 and p3 and the results from all three experiments are shown in the graph below. P₁ P₁ T Identify the CORRECT relationship between these three pressures. Ⓒap₁>P2P2 P3 Oep₁>P2=P3 P₂
The temperature of a constant amount of an ideal gas in a closed system at a fixed pressure, p₁, was increased while the volume was measured. This experiment was repeated twice more at pressures p2 and p3 and the results from all three experiments are shown in the graph below. P₁ P₁ T Identify the CORRECT relationship between these three pressures. Ⓒap₁>P2P2 P3 Oep₁>P2=P3 P₂
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter6: Thermochemisty
Section: Chapter Questions
Problem 6.132QP
Related questions
Question
Transcribed Image Text:----------
The temperature of a constant amount of an ideal gas in a closed system at
a fixed pressure, p₁, was increased while the volume was measured. This
experiment was repeated twice more at pressures p2 and p3 and the
results from all three experiments are shown in the graph below.
P3
P₁
T
Identify the CORRECT relationship between these three pressures.
a. p1>P2 P3
b. p₁ = P2 P3
C. p1 <P2<P3
d. p₁>P2 P3
Oe. p₁ > P2 = P3
Rank the following four substances in order of increasing boiling point.
O a. H₂ C4H10 <HF <H₂O
b. HF <H₂ <H₂O < C4H10
OC. C4H10 <H2 <HF <H₂O
O d. H₂O < HF <H₂ <C4H10
e. H2 C4H10 <H₂O <HF
P₂

Transcribed Image Text:A reaction in a container releases 120 kJ of heat to the surroundings and 50
kJ of work is done on the system by the surroundings. Calculate the change
of internal energy, AU, of the system.
O a. AU = +70 kJ
O b. AU=-70 kJ
C. AU = 0 kJ (no change).
O d. AU +170 kJ
O e. AU = -170 kJ
A 10 g metal bar is heated to 498 K immediately before it is immersed into a
beaker containing 100 mL of water which has an initial measured
temperature of 298 K (the beaker is surrounded by polystyrene to minimize
any loss of heat). After immersion of the hot bar, the temperature of the
water increases to 320 K. A diagram of this experiment is shown below:
Calculate the amount of heat that is transferred from the bar into the water.
(density of water = 1.00 g mL and specific heat capacity of water = 4.18 J
K-1g-1).
O a. +9.20 kJ
O b. +4.18 kJ
O c. –9.20 kJ
O d. +0.920 kJ
O e. -0.920 kJ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning