The theoretical yield of a reaction is the amount of product obtained if the limiting reactant is completely converted to product. Consider the reaction: 2 Fe(s) + 3 Cl₂(g) → 2 FeCl3(s) If 9.920 g Fe is mixed with 16.62 g Cl₂, calculate the theoretical yield (g) of FeCl3 produced by the reaction.
The theoretical yield of a reaction is the amount of product obtained if the limiting reactant is completely converted to product. Consider the reaction: 2 Fe(s) + 3 Cl₂(g) → 2 FeCl3(s) If 9.920 g Fe is mixed with 16.62 g Cl₂, calculate the theoretical yield (g) of FeCl3 produced by the reaction.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter3: Chemical Reactions
Section: Chapter Questions
Problem 121QRT: Ammonia can be formed by a direct reaction of nitrogen and hydrogen. N2(g) + 3 H2(g) 2 NH3(g) A...
Related questions
Question
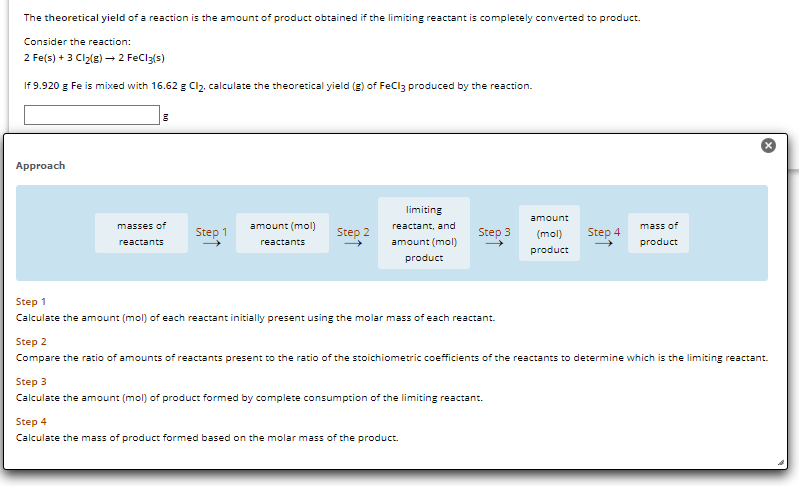
Transcribed Image Text:The theoretical yield of a reaction is the amount of product obtained if the limiting reactant is completely converted to product.
Consider the reaction:
2 Fe(s) + 3 Cl₂(g) → 2 FeCl3(s)
If 9.920 g Fe is mixed with 16.62 g Cl₂, calculate the theoretical yield (g) of FeCl3 produced by the reaction.
Approach
8
masses of
reactants
Step 1
amount (mol)
reactants
Step 2
limiting
reactant, and
amount (mol)
product
Step 3
Step 1
Calculate the amount (mol) of each reactant initially present using the molar mass of each reactant.
Step 4
Calculate the mass of product formed based on the molar mass of the product.
amount
(mol)
product
Step 4
mass of
product
Step 2
Compare the ratio of amounts of reactants present to the ratio of the stoichiometric coefficients of the reactants to determine which is the limiting reactant.
Step 3
Calculate the amount (mol) of product formed by complete consumption of the limiting reactant.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning