The time-averaged potential of a neutral hydrogen atom is given by (1 +%4) ar e b. 4πεο r ar O = where q is the magnitude of the electronic charge, and a Bohr radius. Find the distribution of charge (both continuous and discrete) that will give this potential and interpret your result physically. ao/2, ao being the
Q: A LED lamp is used to do Young’s double slit interference experiment. The slit separation d = 0.02 m...
A: When light passes through narrow slits, the rays bend at the edges and interfere with each, forming ...
Q: a mango of 3 g ia at a certain hight above the ground. the mango has a potential energy of 7J. suppo...
A: Given m=3 g= 0.003 kg g=9.8 m/s^2 PE=mgh=7 J h=7/(9.8*0.003) = 238.095238 m u= initial velocity = 0 ...
Q: Suppose a 51-turn coil lies in the plane of the page in a uniform magnetic field that is directed in...
A: This is a case of changing magnetic flux so Faraday's law will be applied which tells us that Whenev...
Q: You plan to take your hair dryer to Europe,where the electrical outlets put out 240 V instead of the...
A: Given: Power of dryer is 1200 W and voltage is 120 V
Q: Does the wavelength of the wave change if the Frequency changes? Please explain why as well. I am co...
A: We know the frequency (f), speed (v) and wavelength (λ) of a wave have relation
Q: 5. A uniform meterstick weighs 500 dynes. An object of weight 300 dynes is suspended at the 20 - cm ...
A: Center of Mass The Center of mass of a rigid body is that point inside or outside the rigid body whe...
Q: .
A: The state equations are: Rik+1C∫ikdt +Vb =0 ---------(1)Ldikdt +Vk -Vb -1C∫ikdt =0 ...
Q: 1)
A: The volume of the magnesium block is, V=2 m3To find the number of atoms in this volume of the specim...
Q: Given the work functions for some metals:- Aluminum=4.08eV, Beryllium=5.0eV, Cadmium=4.07eV, Calcium...
A: The above problem can be saved by using the concept of photoelectric effect as follows
Q: Two identical rods each of length L and charge Q are perpendicular to each other and separated by a ...
A: Given : Length of the rods = L Charge on the rods = Q Distance = D (is the distance f...
Q: A spaceship from another galaxy passes over the solar system directly above a radial line from the s...
A: The relativistic velocity addition formula between frames S'spaceship and Searth having relative vel...
Q: Using Bohr's model, calculate the velocity of the electron orbiting on a stable trajectory with radi...
A: Using Bohr's model The velocity of the electron orbiting in nth orbit is given by Where z = atomic...
Q: In case of a beam subjected to pure bending about z axis nonzero are: (a) Mx (b) Mz and Vy (c) Nx a...
A: In case of a beam subjected to pure bending about z axis Mz and Vy are non-zero.
Q: The third law of thermodynamics may be stated as “you cannot win, you cannot break even, nor can you...
A: A physicist Snow wittily summarized the third law of thermodynamics as: “You can’t win,” “You can’t...
Q: How can popcorn be made using conduction?
A: Microwave radiation utilizes short, high-frequency waves that penetrate food, which agitates its wa...
Q: Find the electric flux through a single side of regular octahedron of the side a 4cm with the charge...
A:
Q: A large turntable with radius 6.00 m rotates about a fixedvertical axis, making one revolution in 8....
A: If net torque is zero, The angular momenteum of a system is:I0ω0=I2ω2 equation 1At the close...
Q: Consider a race car colliding with the track wall (see figure above). Just before the collision, the...
A: Initial velocity of car, u=90m/s×Cos31i+Sin31ju=77.14i m/s+46.35j m/sThe velocity of car after colli...
Q: A sample of rubidium gas of volume 1 x 10-8 cm3 is cooled until it forms a Bose-Einstein condensate....
A: volume v = 10-8 cm3temp TB = 250n K FIND number of atom in sample
Q: The wavelengths of two components of D-lines of sodium are 5890 Å and 5896 Å, respectively. By how m...
A: The formula to calculate the mean wavelength of the D-lines is given by λ=λ1+λ22.......................
Q: need help with question
A: Part A: Let λR denotes the wavelength of the red light. It is given as 650 nm. The energy differenc...
Q: Subject: Analytical Mechinics
A: IXX =IYY =Izz =23Ma2
Q: Advanced Physics Question
A: (4) Introduction: Simple harmonic motion is defined as a motion in which the restoring force is dire...
Q: "Four charges of equal charge +q are placed at the corners of a rectangle of sides a and b. What is ...
A: Given that,There are four charge +q are placed at the corners of a rectangle of sides a and b,q = 2 ...
Q: (b) A grating having 5000 lines per cm is used under normal incidence of light. What will happen to ...
A: If 90% of the grating is covered then the Number of Slits will decrease and as the It dcreases the ...
Q: A large rock that weighs 164.0 N is suspended from the lower end of a thin wire that is 3.00 m long....
A: Given:- The weight of the rock = 164.0 N Length of the wire =3.00 m Density of the rock ...
Q: (b) A grating having 5000 lines per cm is used under normal incidence of light. What will happen to ...
A: (b) When the 90% of the ruled width of the grating is covered, the angular spacing between the maxim...
Q: For a system of n distinguishable particles to be distributed in two similar compartments, the total...
A: We know, A microstate (W) is a specific configuration of the locations and energies of the atoms or ...
Q: Suppose 1.67 C of charge passes through the filament of a light bulb in 2.00 seconds. How many elect...
A: Amount of Charge Passed = 1.67 C time = 2 sec Current (assumed to be uniform in the filament )=Amoun...
Q: For the diagrams shown below, a wire loop in the plane of the page is moved into or out of a region ...
A: Since we only answer up to 3 sub-parts, we’ll answer the first 3. Please resubmit the question and s...
Q: Simplify/Reduce the system shown in Figure 103 into, T(s)-C(s)/R(s) and solve the natural frequency....
A: please see the next step for solution
Q: Please fill in the blanks
A: Given problem is in the image form:
Q: Three charges, +2.5 µC, −4.8 µC, and −6.3 µC, are located at (−0.20 m, 0.15 m), (0.50 m, −0.35 m), a...
A: Given that,q1=+2.5μC=2.5×10-6 Cq2=-4.8 μC=-4.8×10-6 Cq3=-6.3 μC=-6.3×10-6 CThe electric field at the...
Q: Astronomers observe several galaxies in the Local Group that actually approach the Milky Way. The An...
A: Answer: As per the given statement, Explanation: Galaxies have their own motion in their group...
Q: length period Vlength 2. Turn your intercept into a statement. Hint: "When the is 0, the is ." cm ...
A: Since we only answer up to 3 sub-parts, we’ll answer the first 3. Please resubmit the question and s...
Q: monatomic ideal gas expands slowly to twice its originalvolume, doing 450 J of work in the process. ...
A: Given: W=450 J From first law of thermodynamics, ΔU=Q-W Here, W is work done by the system, 'Q' is h...
Q: (Astronomy) Neutron Star Escape Velocity. What is the escape velocity at the surface of a 2.80-solar...
A: Escape velocity can be calculated by ve=2GMrwhereve is escape velocityG is universal gravitational c...
Q: Suppose a certain person's visual acuity is such that he can see objects clearly that form an image ...
A: Answer: The maximum distance d0=3.28 km
Q: (d) In a Fraunhoffer diffraction due to a narrow slit, calculate the relative intensities of the fir...
A: Since the condition for secondary maxima is, θ=m+12π m is the order of secondary maxima, m = 1,2,3,4...
Q: Find the deflection of the string at any point.
A: Detailed solution is given below:
Q: Q1
A:
Q: If our Sun has a temperature of 6000 K and a luminosity of 1 Solar Luminosity, what is the luminosit...
A: The luminosity of a star is L= R2T4 where L is luminosity R is radius (surface area) ...
Q: ) Calculate the peak wavelength of a black body’s radiation if the black body is at a temperature ...
A:
Q: A 12.48 g sample of an unknown metal, heated to 99.0 °C was then plunged into 50.0 mL of 25.0 °C wat...
A: Given that,12.48 g sample of an unknown metal,Heated to 99.0 °C was then plunged into 50.0 mL of 25....
Q: A uniform drawbridge must be held at a 37° angle abovethe horizontal to allow ships to pass undernea...
A: Under equilibrium condition,∑Fy=0⇒-W7mCos37°+TSin 37°3.5m=0
Q: 1. Calculate the internal energy and heat capacity of the conduction electrons in a block of magnesi...
A: Given that,The volume of the block of magnesium = 2 m3As we know that,The density of magnesium = 174...
Q: Using Bohr's model, calculate the radius of the stable electron orbit with main quantum number 1 for...
A: n= 1 Z= 3
Q: For the given figure, Figure 153, solve for C(s)/R(s) and natural frequency. R(s) C(s) 10 s(s+1) 3s ...
A: please see the next step for solution
Q: A Ti-Saphire laser delivers a 14 ps pulse of 5 MW average power. If the photons have a wavelength of...
A: Detailed solution is given below:
Q: 7. Explain how the Hubble law allows you to estimate the distances to galaxies.
A: According to Hubble's law, the velocity of galaxy with respect to earth is directly proportional to ...
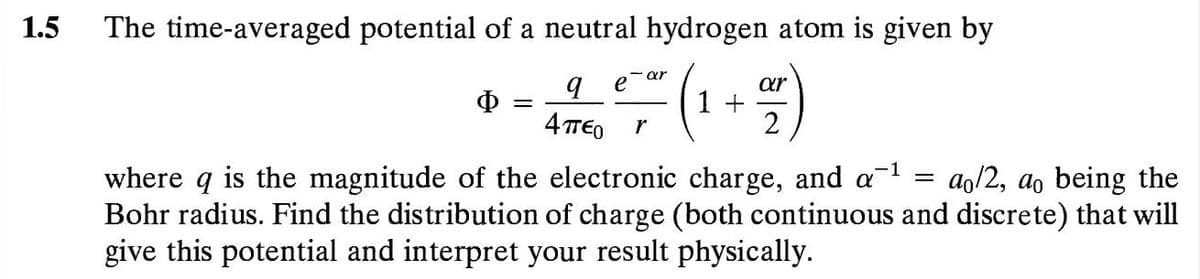
Trending now
This is a popular solution!
Step by step
Solved in 2 steps
